Environmental Engineering Reference
In-Depth Information
Ti(N,O)
Ti(F,O)
Ti(F,N,O)
Ti(C,O)
Ti(S,O)
Anionic
C2
p
S3
p
Ti
3+
Ti
3+
Substitutional
(anionic)
Cationic
N2
p
N2
p
S3
s
C2
p
Ti
3+
C2
p
Interstitial
C-O
·
N-O
·
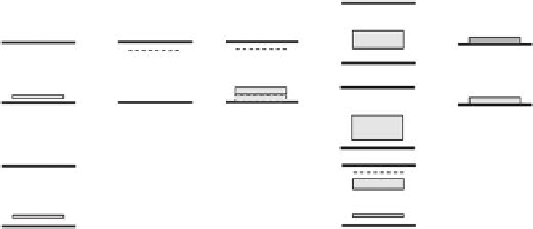
FIGURE 11.7
Electronic effect of N- and/or F-, C-, and S-doping species on the anatase electronic structure. Valence and
conduction bands are represented by their top and bottom edges, respectively. (Adapted with permission
from Kubacka A., Fernández-García M., Colón, G.,
Chem. Rev
., 112, 1555. Copyright 2012, American Chemical
S o c ie t y.)
vacancies. Halogen doping can induce in-gap states near both the VB and the CB and lead to
visible light absorption up to ~800 nm. N-, F-codoped systems exhibit fully occupied N-derived
gap states close to the VB as well as the additional presence of Ti
3+
states near the CB.
Nonmetal doping extends the photosensitive region of semiconductor to visible light.
However, the visible light photoreactivity is highly dependent on the chemical states of
the dopant. A typical example is I-doped TiO
2
. It is found that the I-O-I bond in the doped
TiO
2
can induce a shoulder absorption at 550 nm, while, by combining the I-O-I and the
I-O-Ti bond, the light absorption can be extended to
~
800 nm due to the unoccupied I-O-I
states below the CB edge and the occupied I-O-Ti states above the VB edge.
18
An important case is that the crystalline phase structure can be changed after high-level
doping with anions, such as transformation to oxynitrides, oxysulides, and oxyhalides. The
formed VB consists of N2
p
and S3
p
orbitals, in addition to O2
p
, resulting in signiicant visible
light absorption. For example, compared with Ta
2
O
5
, oxynitride, TaON possesses a narrower
band gap of 2.5 eV and exhibits higher photoreactivity for water splitting under visible light.
19
Codoping with a cation-anion, such as Mo-C-, Ce-C-, Fe-C/N-, V-N-, In-N-, Ce-N-,
Sn-N-, and W-N-doped anatase TiO
2
, have been also studied.
20
One certain contribution
of codoping lies in the handling of the stoichiometry, and makes it possible to control the
defect formation and structural strain. However, only limited theoretical results have been
presented on the synergistic effects of the codoped materials. The structural/electronic
effect needs further analysis to test the potential of cation-anion codoped photomaterials
compared with single-doped materials.
Regardless of metal or nonmetal doping, the localized states formed in the band gap
determine the photosensitive spectral region and redox potential of the photogenerated
charge carriers. However, the visible light absorption induced by doping only makes sense
when the charge carriers created by visible light excitation can supply adequate redox
potential to run the practical photocatalytic reactions. Thus, the challenge is to introduce
suitable dopants to realize the synergistic effect.
11.2.2.3 Self-Doping
Different from impurity incorporation, self-doping (e.g., Ti
3+
doped TiO
2
, oxygen vacancies)
can also provide gap tailoring. These defects can induce an additional shoulder adsorption