Environmental Engineering Reference
In-Depth Information
700,000
Untreated
600,000
500,000
Osorb treated:
0.5% w/v, 30 s
400,000
Osorb treated:
2.0% w/v, 30 s
300,000
200,000
100,000
0
46810
12
14
16
18
20
22
24
26
28
Retention time (min)
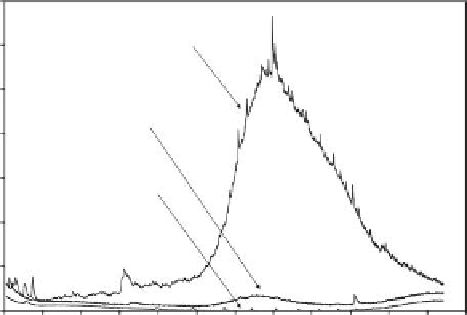
FIGURE 8.9
Gas chromatograms of type B produced water with little pretreatment before and after treatment with 0.5% w/v
and 2.0% w/v Osorb. Measurements were made using the modiied ISO 9377-2 method with detection by mass
spectrometry.
2,000,000
1,800,000
1,600,000
1,400,000
1,200,000
1,000,000
800,000
600,000
400,000
200,000
0
0
Untreated
To luene
Treated
Ethylbenzene
Benzene
Xylenes
Substituted benzenes
1
2
3
4
5
6
7
8
9
Retention time (min)
FIGURE 8.10
Gas chromatograms of type B produced water with little pretreatment before and after treatment with 0.5% w/v
Osorb using selected ion monitoring (
m
/
z
= 91 amu) to detect BTEX compounds.
effect is observed in Osorb. Partitioning occurs within the matrix and swelling is induced,
which leads to exposure of new surface area and pore volume as the nanoporous matrix
expands. Extraction is not dictated by the amount absorbed relative to saturation, but each
organic substance partitions to its own equilibrium as controlled by its polarity and ther-
modynamics of preference to being in a hydrophilic phase or a hydrophobic phase. The
increased absorbance capacity of swelling the media in mixed systems makes the sorbent
potentially useful in produced water treatment owing to the high concentration of mixed
compounds in the water and the need to treat large volumes of water with as little media
as possible.