Environmental Engineering Reference
In-Depth Information
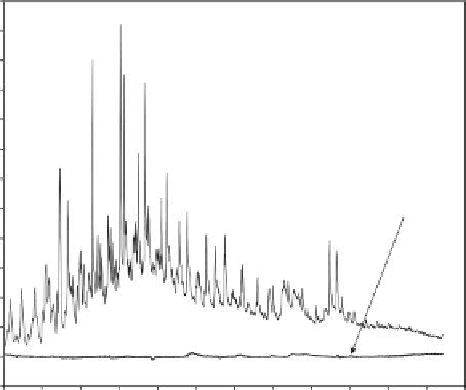
Type A water was well suited for Osorb treatment with >99% of the hydrocarbons
removed with a 30-s application of 0.5% w/v sorbent (Figure 8.8). The rapid and complete
treatment is facilitated by good mass transport dynamics of the hydrocarbons, which all
exist as liquids at the experimental conditions and can readily diffuse into the expanding
nanoporous matrix. It should be noted that particularly high afinity for BTEX compounds
is due to π-π stacking interactions between these sorbates and the aromatic bridging
group of the BTEB precursor. The aromatic bridging groups have been shown to prefer-
entially decorate the outside surfaces of the nanoparticles that comprise the Osorb matrix.
Following extraction, the light fraction of hydrocarbons could be desorbed from the Osorb
at room temperature, indicating that regeneration is facile.
Type B water was more challenging to treat with Osorb in the same amount of time.
The higher molecular weight species are slower to diffuse. In addition, there was likely a
competition between hydrocarbons being adsorbed to suspended particles as opposed to
partitioning into Osorb. Finally, the water held approximately twice the organic content of
the preprocessed type A water. As a result, four times the amount of Osorb (2% w/v) was
required to achieve a >99% level of organic removal within a 30 s treatment time (Figure
8.9). Although the organic content of type B water is dominated by higher molecular spe-
cies, a substantial concentration of the more soluble aromatic components is also present
in type B water. The amount of BTEX in treated and untreated water was analyzed sepa-
rately using mass spectrometry to selectivity detect these analytes. The gas chromatog-
raphy-mass spectrometry results show >99.9% of BTEX was removed concomitant with
heavy petroleum removal (Figure 8.10). Co-removal of mixed contaminants is typical of
Osorb, which is facilitated by the ability to swell. Speciically, absorption of one compound
does not hinder the absorption of another. In contrast, absorption typically improves in a
mixed-contaminant stream. Such behavior is unusual for standard adsorbents where par-
titioning occurs between water and active sites on a surface or inite number of molecular-
scale pores. As sites saturate, the capacity of the absorbent rapidly declines. The opposite
550,000
500,000
450,000
400,000
350,000
300,000
250,000
200,000
150,000
100,000
50,000
Untreated
Treated
0
-50,000
46810
12
14
16
18
20
22
24
26
28
Retention time (min)
FIGURE 8.8
Gas chromatograms of type A pretreated produced water before and after treatment with 0.5% w/v Osorb.
Measurements were made using the modiied ISO 9377-2 method with detection by mass spectrometry.