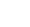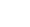Environmental Engineering Reference
In-Depth Information
(a)
(b)
(c)
(d)
(e)
(40×)
(100×)
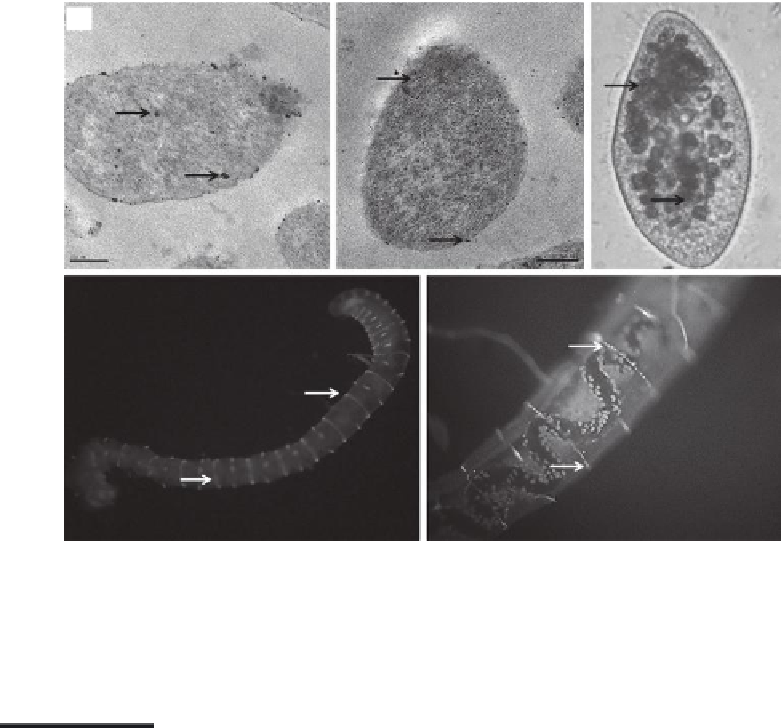
FIGURE 5.2
Internalization of the nanoparticles in different aquatic organisms: (a, b)
Escherichia coli
, (c)
Paramecium caudatum
,
and
(d, e)
Tubifex tubifex
.
5.3 Approaches and Knowledge Gaps in Aquatic Toxicity Studies
The frequent release and interaction of ENPs with different components of the aquatic eco-
system necessitates the development of certain strategies to test their possible hazards. It
can be inferred from the current understanding of fate, behavior, and detection of different
ENPs in the ecosystem that interactions of cells with ENPs are dependent on size, shape,
chemical composition, surface charge, surface structure, area, solubility, and agglomera-
tion. Hence, it is essential to study these physiochemical properties of ENPs, both in dry as
well as in suspension for hazard assessment [36].
Among these physiochemical characteristics, the surface properties of the ENPs are the
most important factor that governs the stability and mobility of ENPs in aqueous suspen-
sion [36]. The agglomeration tendency of the ENPs is determined by the properties such as
surface area, functionalization, temperature, ionic strength, pH, concentration, size, and
the solvent [45]. However, it is dificult to measure the surface properties of ENPs at the
nanogram to picogram range because of the limitations of the commercially available ana-
lytical instruments. On the other hand, the concentration of ENPs in suspension is also a
crucial step in designing the experiments. Since the ENPs have a tendency to agglomerate/
aggregate, it results in a change in their physicochemical properties, and hence the avail-
able cellular concentration [46]. Thus, the experimental design should also consider the
concentration-induced aggregation effects of the ENPs.