Environmental Engineering Reference
In-Depth Information
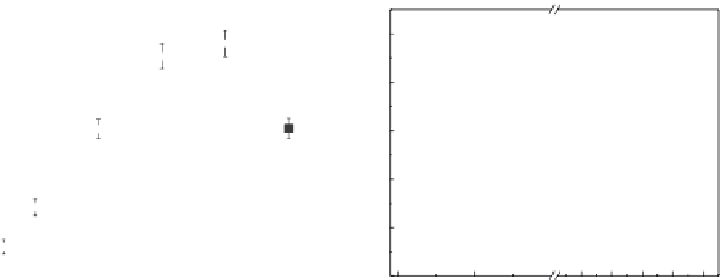
Parshetti and Doong [148] immobilized bimetallic Ni/Fe in PEG/PVDF and PEG/nylon
66 membranes for dechlorination of TCE. SEM images and electron probe microanalysis
(EPMA) elemental maps showed that the distribution of Fe in the nylon 66 membrane was
uniform and the intensity of Ni layer was higher than that in PVDF membrane (Figure
4.4a through d). The particle sizes of bimetallic Fe/Ni in PVDF and nylon 66 membranes
were 81 ± 12 and 55 ± 14 nm with the Ni layers of 12 ± 3 and 15 ± 2 nm, respectively. The
eficiency and rate of TCE dechlorination increased upon increasing the mass loading of
Fe
LvArea%
(a)
(c)
0.0
0.0
0.2
0.8
2.9
6.7
10.4
14.2
14.9
13.8
12.1
9.4
7.5
4.7
1.9
0.4
0.0
0.0
351
331
311
291
271
251
232
212
192
172
152
133
113
93
73
53
34
190
10 µm
10 µm
Ave
Ni
LvArea%
(b)
(d)
103
0.0
0.0
0.0
0.0
0.0
0.0
0.0
0.0
0.0
0.0
0.0
0.0
0.1
0.2
1.1
15.9
82.6
0.0
96
90
83
77
70
64
57
51
45
38
32
25
19
12
6
0
4
10 µm
10 µm
Ave
(e)
(f)
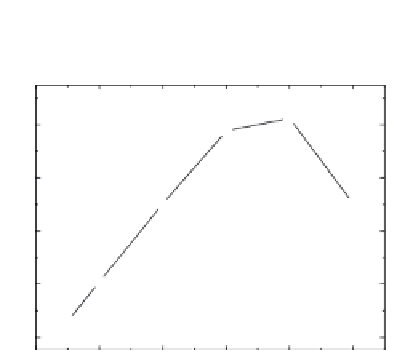
1.0
8
Particles were
stored for 48 h
without TCE
addition.
Similar process
followed after
each cycle
onward
0.8
7
0.6
6
0.4
5
0.2
4
0.0
0
5
10
15
20
25
0
5
50
100150 200250
Ni loading (wt %)
Time (h)
FIGURE 4.4
EPMA elemental maps of Ni and Fe species in microiltration membranes. (a) Fe in PVDF membrane, (b) Ni in
PVDF membrane, (c) Fe in nylon 66 membrane, and (d) Ni in nylon 66 membrane. (e) The pseudo-irst-order rate
constant for TCE dechlorination as a function of mass loading of nickel. (f) Stability and durability of Fe/Ni
nanoparticles in nylon 66 membrane for TCE dechlorination. The total mass of Fe/Ni used was 7.2 mg. (Cited
from Parshetti GK, Doong RA,
Water Res
, 43, 3086,
20 09.)