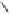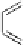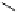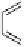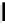Biology Reference
In-Depth Information
in vitro
mimicry of the non-enzymatic yet diastereoselective formation of
acutissimin flavano-ellagitannins.
+H
+
/-H
2
O
1
[
27
]
OH
8
HO
O
OH
-H
+
6
R
retention of
configuration
at C-1
OH
R =
β
-OH, (+)-catechin
R =
α
-OH, (-)-epicatechin
HO
HO
OH
OH
HO
HO
HO
HO
OH
OH
R
O
O
HO
HO
HO
O
O
OH
O
O
O
HO
HO
O
R
OH
6'
O
O
O
HO
8'
1
OH
O
O
O
OH
HO
O
O
1
HO
O
+
O
O
O
OH
HO
HO
14
/
15
: 75:25 (87%)
29
/
30
: 67:33 (78%)
O
OH
O
HO
OH
HO
OH
HO
OH
HO
HO
OH
HO
OH
15
: R =
β
-OH, (-)-acutissimin B
30
: R =
α
-OH, (-)-epiacutissimin B
HO
14
: R =
β
-OH, (-)-acutissimin A
29
: R =
α
-OH, (-)-epiacutissimin A
Fig. 9.10 Hemisynthesis of acutissimins (
14
/
15
) and epiacutissimins (
29
/
30
) from
vescalagin (
1
) and either (+)-catechin or (-)-epicatechin, respectively, in acidic organic
media (isolated yields, see text).
9.3 Impact of Vescalagin on the Chemical Profile of Wine
9.3.1 Occurrence of flavano-ellagitannins in red wine
Several hundred compounds present in red wine have been characterized
to date, so the chances of finding the acutissimins in such a chemically
complex medium would have been small without the compounds already
to hand, thanks to our hemisynthesis work. On the one hand, we first
verified that the oak heartwood used to make barrels and from which we
isolated vescalagin (
1
) (Quideau
et al.
, 2004, Scalbert
et al.
, 1990), did