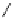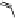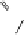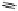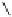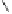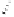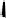Biology Reference
In-Depth Information
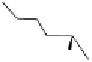
To date the largest identified oligomeric
C
-glycosidic ellagitannin is
a vescalagin/castalagin-based pentamer, called castaneanin D (
11
, Fig.
9.3), which has been isolated (and characterized) by Kouno, Tanaka and
co-workers (Tanaka
et al.
, 1996) from the heartwood of the Japanese
chestnut tree (
i.e.
,
Castanea crenata
S
IEB
.
et
Z
UCC
., Japanese name:
Kuri).
HO
HO
HO
HO
OH
HO
HO
HO
OH
HO
HO
O
O
O
O
HO
O
O
O
O
HO
O
O
O
HO
O
O
OH
H
HO
O
OH
O
O
O
O
O
HO
O
OH
HO
HO
OH
HO
OH
HO
OH
OH
OH
HO
HO
n
OH
Fig. 9.3 Castaneanin D, the largest oligomeric
C
-glycosidic ellagitannin known to date.
11
: castaneanin D (
n
= 4)
9.1.1.2
Complex C-glycosidic ellagitannins
The
C
-glycosidic ellagitannin subclass also encompasses so-called
complex tannins, which are structural hybrids composed, in their
simplest variations, of a
C
-glycosidic ellagitannin moiety derived for
example from the monomers vescalagin (
1
) or stachyurin (
3
) and a
flavan-3-ol unit such as catechin or epicatechin. In these complex
tannins, both parts are connected via a C-C linkage between the carbon-1
center of the open-chain glucose core of the ellagitannin moiety and
either the carbon-8 or the carbon-6 center of the ring-A of the flavan-3-ol
unit. Depending on the nature of each moiety, the regiochemistry of
attachment to each other, and the type of bond connectivities through
which each moiety can lead to oligomeric variants, complex tannins
further contribute to the ellagitannin structural diversity. Their natural
occurrence appears to be limited to plant species of the families
Fagaceae
,
Combretaceae
,
Myrtaceae
,
Theaceae
and
Melastomataceae
(Yoshida
et al.
, 1992).