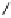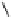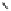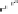Biology Reference
In-Depth Information
membered hemiacetal or acetal rings. Geraniin, in its crystalline form,
adopts the six-membered hemiacetal ring form, but equilibrates back into
a mixture of both cyclic hemiacetals or acetals when dissolved in an
aqueous or an alcoholic solution. This behavior is reminiscent of that
observed for
D
-fructose, which adopts a cyclic pyranose structure in the
crystalline state, but equilibrates between fructopyranose and
fructofuranose in aqueous solutions (Okuda
et al
., 1982a).
O
O
O
O
O
HO
OH
O
HO
OH
OH
OH
OH
HO
HO
OH
HO
OH
HO
OH
O
Galloyl
HHDP
DHHDP
O
O
HOOC
HO
OH
OH
O
Transformed DHHDP
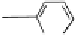
O
Fig. 1.1 Oxidative transformations from the galloyl and HHDP groups.
Further oxidative transformations of the DHHDP group yield several
other subclasses of ellagitannins, some members of which are shown in
the following part of this chapter and in Chapter 2.
1.3.2 Regiospecificity of the HHDP group on the glucose core, and
its correlation to plant families
The positioning of the HHDP group or its oxidized variants on the
glucose core is generally the same in ellagitannins produced by plant
species of the same family. Thus, one type of ellagitannins bears the
HHDP group at their glucose O-2~O-4 and/or O-3~O-6 positions, and
another type bears it at their O-2~O-3 and/or O-4~O-6 positions.
Ellagitannins of the former type such as geraniin, corilagin and granatin
B are produced by plants of the
Geraniaceae
,
Combretaceae
and
Punicaceae
families, as well as in most species of euphorbiaceous plants.
The latter type of ellagitannins exemplified by pedunculagin and