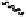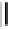Biology Reference
In-Depth Information
were responsible for receptor recognition and binding, and if the
digalloyl ether linker unit of
81
was complicit in receptor component
organization, then altering the latter moiety might meet the requirements
of binding without activation. That is, the hypothesis that a change in the
spacing and orientation of this linker could bring about a substantial
decrease (or increase?) in the ability of the receptor components to
interact, was tested.
6.5.2.1
In vitro testing with hPBMC's
Five different dimeric gallotannin/ellagitannin analogues were
synthesized based on model dimer
81
through coupling of the
appropriate diacid chlorides and two molecules of tetragalloylated
glucopyranose (Fig. 6.23) (Feldman
et al.
, 2002). Analogue
82
,
possessing the most conservative alteration (digalloyl ether hydroxyls
removed), was used to test the importance of the phenolic groups in the
otherwise identical linker of the parent
81
. Derivative
83
featured a more
lipophilic and flexible linker. Analogues
84
-
86
probed the consequences
of modifying both the orientation and spacing of the linker.
OH
O
GO
O
O
OG
OG
GO
Linker
OH
G =
O
O
O
GO
GO
GO
O
GO
OH
dimeric analogues gallotannin/ellagitannin hybrids
O
Linker
=
83
84
82
85
86
Fig. 6.23 Dimeric gallotannin analogues
82
-
86
of model dimer
81
.
Derivatives
82
-
86
were individually and independently added to
hPBMC's and assayed for their ability to generate TNFα at the 24-hour