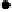Biology Reference
In-Depth Information
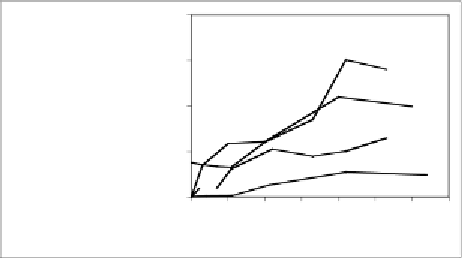
potent at generating TNFα from hPBMC's than either of the dimers
62
or
81
at similar concentrations (Fig. 6.17). This observation correlates
well with the very different anticancer profiles (Miyamoto
et al.
, 1987b)
between coriariin A (3 out of 6 regressors and a 238 %ILS) and β-PGG
(zero regressors and 82 %ILS) reported by Miyamoto. Since a measure
of IL-1β secretion amounts did not reveal a similar correlation, it
remains unlikely that IL-1β is the primary mediator responsible for the
tumoricidal activities of tannins. It is also noteworthy that the TNFα-
inducing abilities of coriariin A and its model analogue
81
are similar
(Fig. 6.17). These data show that the HHDP group is not necessary for
activity and that two unconnected galloyl groups can take its place,
which is consistent with Miyamoto's
in vivo
SAR studies of ellagitannins
against sarcoma-180 (Miyamoto
et al.
, 1987b).
4
3
TNF
α
(ng/mL
2
Coriariin A 62
PGG 66
Model Dimer 81
LPS
1
0
0
5
10
15
20
25
30
35
Conc of Tannins (
μ
M) & LPS (
μ
g
/mL)
Fig. 6.17 Dose dependence profile of TNFα release (24 hours) upon hPBMC stimulation
by several tannins and LPS.
Recently, other hydrolyzable tannins have been reported to
effectively modulate the release of TNFα from
Leishmania donovani
infected macrophages (RAW 264.7)
in vitro
(Kolodziej
et al.
, 2001).
In
total, 27 tannins were tested including agrimoniin, tellimagrandin II,
gallic acid, and β-PGG. Contrary to the above results, it was reported
that oligomeric ellagitannins (EC
50
>25 μg/mL) were less potent as TNF
inducers (both TNFα and β) than monomeric ellagitannins and
gallotannins (EC
50
8.5 to >25 μg/mL), and
C
-glycosidic ellagitannins and
dehydroellagitannins (EC
50
0.6-2.8 μg/mL). In these experiments, TNFα