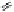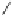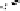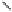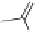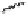Biology Reference
In-Depth Information
reversed phase thin layer chromatography (Khanbabaee and Großer,
2003).
Access to the key intermediate
90
was then re-examined in the aim
of shortening the overall synthesis. In particular, the possibility of
making it in one step by a direct acylation of tetrol
88
with (
S
)-
16
was
explored. Remarkably, this reaction promoted under Steglich conditions
using DCC and DMAP led to the formation of only one unpolar product,
which could easily be identified as the tetraester
90
by comparing its
characterization data with those of the same compound previously made
via
the stepwise strategy (Khanbabaee and Großer, 2003).
HO
O
Ph
O
O
HO
O
R
R
HO
HO
HO
HO
1. (
S
)-
16
, DCC,
DMAP
2. HCl
88
: R = OBn(
o
-nitro)
2
: R = OBn(
o
-nitro)
(
S
)-
16
, DCC,
DMAP
HO
BnO
O
OBn
HO
R
O
BnO
2
BnO
3
O
O
BnO
O
BnO
S
(
S
)-
16
BnO
S
89
O
O
DCC, DMAP
6
BnO
OBn
O
BnO
O
BnO
O
4
R
O
BnO
BnO
2
3
O
O
BnO
O
BnO
S
HO
OH
BnO
OBn
BnO
HO
90
HO
O
O
Pd/C, H
2
6
O
HO
O
O
4
OH
O
HO
HO
2
3
O
O
1
HO
O
HO
87
HO
OH
HO
Fig. 5.18
Total synthesis of pedunculagin (
87
) via the double esterification strategy.