Biology Reference
In-Depth Information
The second building block
52
was synthesised starting from
commercially available benzylidene acetal
51
by acylation with 3,4,5-
trimethylgallic acid
54
, followed by the removal of the benzylidene
acetal with Pd(OH)
2
in cyclohexane. Esterification of (
S
)
-
50
with the
resulting
D
-glucopyranosyl derivative, methyl 2,3
-
di
-
O
-
(3,4,5
-
tri
-
O
-
methylgalloyl)
-
α
-
D
-
glucopyranose (
52
) yielded the permethylated
tellimagrandin I (
53
, Fig. 5.10).
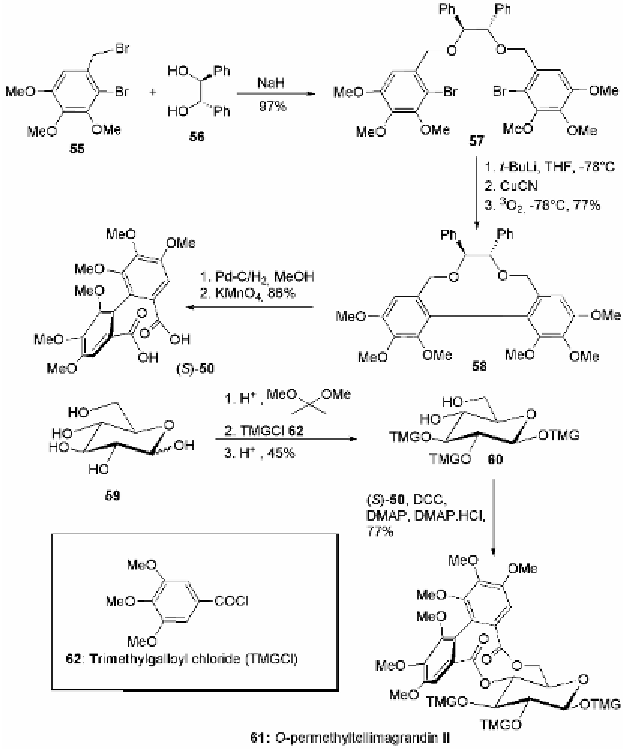
Fig. 5.11 Synthesis of
O
-permethyltellimagrandin II (
61
).