Biology Reference
In-Depth Information
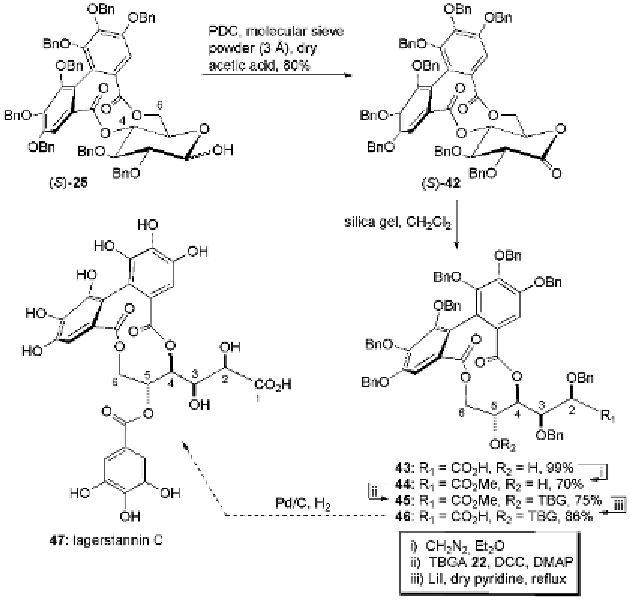
no avail. However, once the carboxylic acid
43
was protected as its
methyl ester
44
using a freshly prepared diazomethane solution,
acylation with 3,4,5-tri-
O
-benzylgallic acid
22
under standard Steglich
conditions (Neises and Steglich, 1978, Höfle
et al.
, 1978) then yielded
the desired product
45
(Fig. 5.9).
Fig. 5.9
Synthesis of undeca-
O
-benzyllagerstannin C (
46
).
It is known that methyl esters can be cleaved selectively under mild
conditions using strong nucleophiles in dipolar, aprotic solvents (
i.e.
,
carboxylate exchange by S
N
2 dealkylation). Other carboxylic esters of
more sterically demanding alcohols (even ethanol) that may be present in
the molecule are not at all, or only very slowly, attacked under such
conditions (Meinwald and Putzig, 1970, Dean, 1965, Borch
et al.
, 1972,