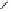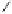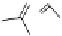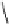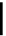Biology Reference
In-Depth Information
respectively. Gemin D (
37
, Yoshida
et al.
, 1982, 1985, Hatano
et al.
,
1988, Lee
et al.
, 1989) and hippomanin A (
38
, Rao, 1974, 1977, Hatano
et al.
, 1988, Lee
et al.
, 1989) were finally obtained by complete
debenzylation under standard hydrogenolysis conditions (Fig. 5.7)
(Khanbabaee
et al.
, 1999). The spectroscopic data of synthetic
37
agreed
in all respects with those of natural
37
(Yoshida
et al.
, 1985). The
complete NMR data of the natural product
38
were published in 1999 for
the first time (Khanbabaee
et al.
, 1999).
O
Ph
O
O
OBn
HO
HO
28
BnBr,
n
-Bu
4
NI,
0.68
M
NaOH, 74%
29
:
30
= 3:1ratio
O
O
Ph
Ph
O
O
HO
O
BnO
O
+
OBn
OBn
BnO
29
HO
30
TBGA
22
,
DCC, DMAP
R
1
R
1
O
O
R
2
R
2
OBn
OBn
+
BnO
TBGO
BnO
TBGO
31
: R
1,
R
2
= CHPh, 60%
33
: R
1
= R
2
= OH, 80%
34
: R
1,
R
2
= (
S
)-HBDP, 68%
32
: R
1,
R
2
= CHPh, 25%
35
: R
1
= R
2
= OH, 81%
36
: R
1,
R
2
= (
S
)-HBDP, 66%
i
i
ii
ii
i) 2 N HCl
ii)
rac
-
16
,
DCC, DMAP
Pd/C, H
2,
89%
Pd/C, H
2,
88%
HO
HO
OH
OH
HO
HO
HO
HO
O
O
O
O
6
6
HO
O
HO
O
O
O
O
O
4
4
HO
HO
GO
O
H
HO
O
H
HO
GO
37
: gemin D
38
: hippomanin A
Fig. 5.7 Total synthesis of gemin D (
37
) and hippomannin A (
38
).