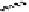Biology Reference
In-Depth Information
For the development of a strategy for the synthesis of strictinin (
27
),
two major points were to be addressed: the atropisomerism and the
β
-
configuration of the anomeric center of the
D
-glucopyranose core. The
two key building blocks needed for this total synthesis according to
method
A
(see Fig. 5.1) were the
ortho
-nitrobenzylated 2,3-di-
O
-benzyl-
β-
D
-glucopyranose
23
, available in four steps (Feldman and
Sambandam, 1995, Zehavi, 1988, Zehavi and Patchornic, 1972, Zehavi
et al.
, 1972, Igarashi, 1977, Königs and Knorr, 1901, Rajasekharan
Pillai, 1980, Gigg
et al.
, 1983, Kanai
et al.
, 1987, Czernecki
et al.
, 1976),
and the racemic hexabenzyloxydiphenic acid
rac
-
16
(Schmidt
et al.
,
1954).
OBn
OBn
BnO
OBn
HO
DCC, DMAP,
CH
2
Cl
2,
reflux,
BnO
O
HO
BnO
CO
2
H
CO
2
H
OBn
R
+
polar products
BnO
+
BnO
32%
O
BnO
O
6
23
: R = OBn(
o
-NO
2
)
BnO
O
BnO
O
O
4
OBn
OBn
BnO
R
rac
-
16
OBn
(
S
)-
24
:
R =
β
-OBn(
o
-NO
2
)
(S)-
25
: R =
α
,
β
-OH, 90%
(S)-
26
: R =
β
-OTBG, 88%
i
i) h
ν
, THF
ii) TBGCl
7
, Et
3
N
ii
HO
Pd/C, H
2
79%
OH
HO
HO
O
OH
O
6
HO
O
OH
O
O
4
HO
O
HO
OH
HO
O
27
: strictinin
Fig. 5.6 Total synthesis of strictinin (
27
).
The total synthesis of strictinin (
27
) commenced with the double
esterification of
rac
-
16
using diol
23
under Steglich conditions (Neises
and Steglich, 1978, Höfle
et al.
, 1978) to furnish the (
S
)-configured
esterification product
24
in a completely diastereoselective manner (Fig.
5.6). The (
R
)-hexabenzyloxydiphenic acid
(R)
-
16
present in the racemic