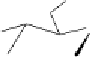Biology Reference
In-Depth Information
(
S
)-
20
(
R
)-
20
TBGCl
7
,
Et
3
N, 66%
TBGCl
7
,
Et
3
N, 69%
OTBG
OTBG
O
TBGO
O
TBGO
OTBG
O
O
OTBG
BnO
BnO
O
O
O
O
BnO
BnO
O
O
BnO
BnO
BnO
BnO
OBn
OBn
BnO
BnO
(
S
)
-21
(
R
)
-21
Pd/C, H
2,
85%
Pd/C, H
2,
85%
OG
OG
O
O
GO
GO
OG
O
OG
O
HO
HO
O
O
O
O
HO
HO
O
O
HO
(
R
)
HO
HO
HO
OH
OH
HO
HO
(
S
)-
14
:
pterocaryanin C
(
R
)-
12
:
mahtabin A (cercidinin A)
OR
1
OR
R
1
O
RO
OR
1
OR
R
2
O
O
R = Bn, tribenzylgalloyl (TBG)
R = H, galloyl (G)
22
: R
1
= Bn, R
2
= OH, tribenzylgallic acid (TBGA)
7
: R
1
= Bn, R
2
= Cl, tribenzylgalloyl chloride (TBGCl)
Fig. 5.4 Total synthesis of mahtabin A (
12
) and pariin M (
13
) and their
diastereoisomeric counterparts, pterocaryanin C (
14)
and praecoxin B (
15
).
The total synthesis of all four diastereoisomeric ellagitannins
12
-
15
started from the 4,6-benzylidene acetal-protected
D
-glucopyranose
derivative
2
, which was first acylated with racemic
hexabenzyloxydiphenic acid
rac
-
16
to give the two diastereoisomers
(
S
)-
17
and (
R
)-
17
(Fig. 5.4). The absolute configuration of the biaryl
component in (
S
)-
17
and (
R
)-
17
could be determined after ester
hydrolysis by using the Gassman method (Gassman and Schenk, 1977).
The so obtained optically pure hexabenzyloxydiphenic acids (
S
)-
16
and