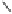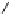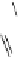Biology Reference
In-Depth Information
R
2
O
OR
1
R
2
O
OR
1
R
3
O
HO
R
3
O
+
O
R
3
O
HO
Method A
R
3
O
R
6
O
OR
4
O
O
6
R
5
O
O
R
2
O
O
OH
4
O
II
O
R
2
O
O
OH
R
1
O
OR
4
R
6
O
R
1
O
I
R
5
O
R
2
O
III
OR
1
R
2
O
R
3
O
OR
1
X
R
3
O
R
3
O
X
O
Method B
+
II
O
O
X
R
2
O
O
OH
O
O
OR
4
R
6
O
R
1
O
IV
: X = H or I
V
R
5
O
Fig. 5.1
Two strategies for the construction of the ellagitannin framework.
There are different ways to access the protected
hexahydroxydiphenic (HHDP) acid that is a pre-requisite for method
A
.
For example, as we shall discuss later in this chapter, the synthesis of
the enantiopure (
S
)-hexamethoxydiphenic acid (
S
)-
50
has been
accomplished by enantioselective reductive coupling reactions between
chiral aryl components either in an intermolecular (Nelson and Meyers,
1994) or in an intramolecular fashion (Lipshutz
et al.
, 1993, 1994a/b).
The subsequent esterification of the enantiopure (
S
)-
50
with a suitably
functionalised diol derivative of
D
-glucopyranose opened the way for the
synthesis of permethylated ellagitannins (see Fig. 5.10 and 5.11). A
different route to the stereoselective synthesis of this type of
permethylated ellagitannins is provided by application of the concept of
kinetic racemate resolution through which, for example, the racemic
mixture of hexamethoxydiphenoyl dichloride
rac
-
80
(see Fig. 5.16)
serves to bisacylate a suitably functionalised diol derivative of
D
-
glucopyranose (Itoh and Chika, 1995, Itoh
et al.
, 1996).
However, due to the dearth of suitable methods for the cleavage of
the methyl ether groups of the permethylated precursor of natural
ellagitannins, the total syntheses of the corresponding ellagitannins could
not be successfully completed (Nelson and Meyers, 1994, Lipshutz
et al.
,