Biology Reference
In-Depth Information
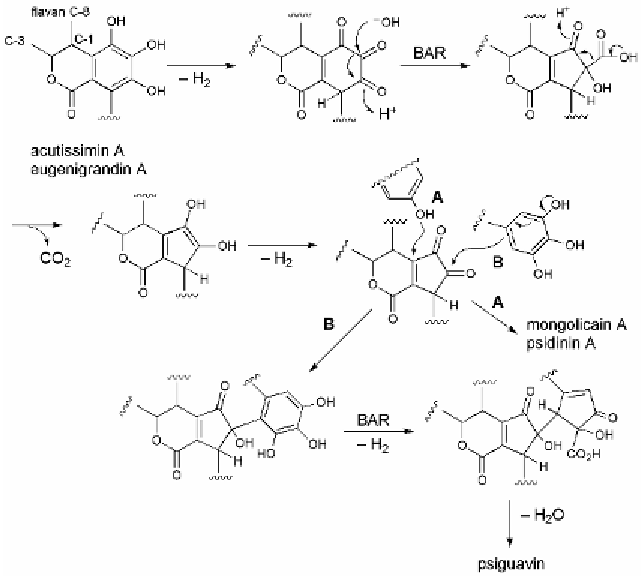
the case of complex ellagitannins comprising flavan-3-ol units.
Acutissimin A (Ishimaru
et al.
, 1987) and eugenigrandin A (Lin
et al.
,
1991) have (+)-catechin and (+)-gallocatechin units, respectively, at the
vescalagin unit C-1 position, and these tannins are usually accompanied
by their oxidized form, mongolicain A (Nonaka
et al.
, 1988) and psidinin
A (Tanaka
et al.
, 1992c), respectively (Fig. 4.18). The cyclopentenone
ring attached to the glucose C-1 position of these tannins is generated by
oxidation of the pyrogallol ring, followed by a benzylic acid-type
rearrangement
(BAR)
and
subsequent
decarboxylation
and
dehydrogenation (Fig. 4.19).
Fig. 4.19 Oxidative metabolism of
C
-glycosidic ellagitannins.
Furthermore, psiguavin isolated from guava (Tanaka
et al.
, 1992c)
has an even more complex structure. The B-ring of the gallocatechin unit