Biology Reference
In-Depth Information
In the course of chemical studies on dehydroellagitannins, acetone
adducts are sometimes isolated as artifacts of the extraction procedure
using aqueous acetone. The formation of these acetone adducts of the
DHHDP units has nevertheless some benefits, because these adducts are
sufficiently stable as compared to the phenazine derivatives. Moreover,
with the exception of the signals due to CH
2
COCH
3
, the NMR spectra
closely resembled those of the five-membered ring hemiacetal structures.
Furthermore, the absence of the additional aromatic ring renders the
13
C-
NMR spectra much simpler than those of the phenazine derivatives.
The addition of acetone to DHHDP bisester groups is efficiently
catalyzed by ammonium ions; an alternative and simple derivatization
method was thus developed. An example applied to euphorscopin is
shown in Fig. 4.15 (Lee
et al.
, 1991, Tanaka
et al.
, 1992a). This
derivatization is very simple: ammonium formate is added as a catalyst
to an aqueous acetone solution of tannins or plant extracts that is left
standing at room temperature overnight or at 50 °C for 1 to 2 h.
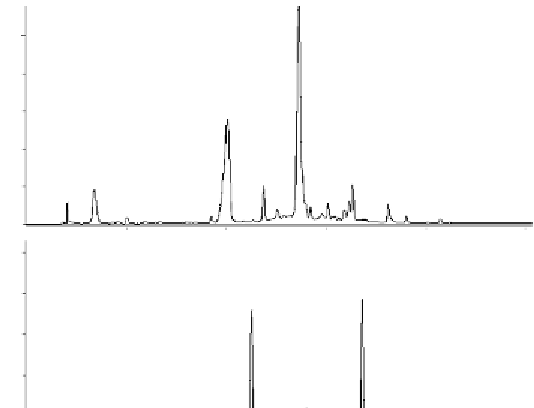
granatin B
A
granatin A
10.0
20.0
30.0
40.0
50.0 (min)
B
acetone adduct
of granatin B
acetone adduct
of granatin A
10.0
20.0
30.0
40.0
50.0 (min)
Fig. 4.16 HPLC analyses (220 nm) of the leaves of
Punica granatum
.
A
: aqueous
acetone extract;
B
: aqueous acetone extract treated with HCOONH
4
.