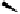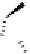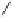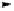Biology Reference
In-Depth Information
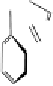
4.3.4 Application of the reactions of the DHHDP bisester
As mentioned before, the DHHDP bisester group exists as an
equilibrium mixture of two hemiacetal structures, and this phenomenon
sometimes increases the difficulty of spectral interpretation and HPLC
analysis. Moreover, in alcoholic solutions, the acetal hydroxyl groups are
substituted by the alcohol molecules, such that the mixture becomes even
more complex. To avoid this complexity, it is necessary to convert the
DHHDP units into phenazine derivatives by condensation with
o
-
phenylenediamine (Schmidt
et al.
, 1967a, Okuda
et al.
, 1982, see also
Chapter 1). The NMR signals of the resulting sugar moieties are largely
shifted after derivatization, because the anisotropic effect of the
phenazine ring caused significant up-field shifts of the sugar proton
signals. These properties are often useful, because the observed chemical
shift changes correlate to the stereochemistry of the molecule. However,
the derivatives are usually unstable and gradually hydrolyzed in solution.
O
H
3
C
C
H
2
OH
O
OH
OH
HO
O
HO
O
OH
HO
O
OH
OH
HO
H
H
HO
acetone
HCOONH
4
O
O
O
O
O
O
O
HO
O
O
O
HO
euphorscopin
O
OH
O
OG
HO
O
OH
acetone adduct
HO
HO
OH
OH
OH
NH
2
O
H
N
HO
OH
OH
HO
O
O
O
O
N
OH
HO
NH
2
O
O
OH
O
OH
HO
O
HO
O
O
OH
O
O
HO
O
O
HO
O
O
OH
O
HO
HO
O
O
OG
HO
phenazine derivative
HO
Fig. 4.15 Formation of phenazine and acetone adducts of euphorscopin.