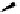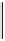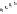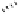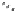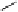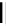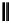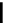Biology Reference
In-Depth Information
(Nonaka
et al.
, 1983, Hashimoto
et al.
, 1988). Enzymatic oxidation of
(-)-epigallocatechin-3-
O
-gallate affords theasinensin A, which possesses
a structure similar to that of the ellagitannin HHDP group (Hashimoto
et
al.
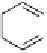
, 1992). However, the production of the catechin-derived dimer does
not occur through direct coupling of the pyrogallol rings. Instead,
epigallocatechin is first oxidized to give a B-ring
ortho
-quinone, then
dimerization spontaneously and stereoselectively occurs to give
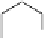
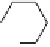
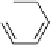
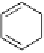
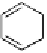
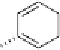
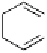
dehydrotheasinensin A (Fig. 4.12) (Tanaka
et al.
, 2002, 2003).
OH
OH
OH
O
- H
2
HO
O
HO
O
OH
O
OG
OG
(-)-epigallocatechin-3-
O
-gallate
OH
OH
- H
2
, + H
2
O
OH
OG
OH
HO
HO
O
H
H
OH
O
H
OH
O
HO
HO
OG
OH
dehydrotheasinensin A
O
pH 7.0
r.t.
OH
HO
GO
GO
OH
HO
HO
HO
H
O
O
OH
H
O
+
OH
O
H
CO
2
H
H
OH
*
HO
OH
OH
HO
O
HO
O
OG
OH
O
reduction products
theasinensin A
⇒ *
(
R)-
biphenyl bond
theasinensin D
⇒ *
(
S)-
biphenyl bond
OG
oxidation product
OH
Fig. 4.12 Oxidation of epigallocatechin and degradation of dehydrotheasinensin A.