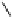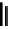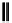Biology Reference
In-Depth Information
OH
HO
O
OH
HO
HO
HO
O
O
O
OH
O
O
O
HO
HO
O
O
O
O
OH
HO
OH
O
O
O
O
O
O
O
O
OH
O
OH
HO
O
O
O
HO
OH
OH
OH
HO
HO
OH
OH
HO
OH
21
HO
HO
OH
OH
The acylated glucopyranose ring of pentagalloylglucose (PGG,
8
) is
strongly hydrophobic and the anomeric position is the most sterically
unhindered site; therefore, the hydrophobic benzoyl group of
paeoniflorin selectively associates with the anomeric position of
8
(Fig.
4.4). The association of paeoniflorin with
8
was also visualized by
dissolution of precipitates of the caffeine-pentagalloylglucose complex
by the addition of paeoniflorin (Cai
et al.
, 1990).
Fig. 4.3 Gel formation of pentagalloylglucose (PGG,
8
) in an aqueous solution and
dissolution by addition of paeoniflorin; PG: pentagalloylglucose (10mg/ml), PAE:
paeoniflorin (10mg/ml), Suc: sucrose (17mg/ml), tannic acid (20mg/ml)
Tannic acid, a mixture of polygalloylated glucoses bearing additional
depsidically-linked galloyl groups, does not form any gel. This is
probably because the additional galloyl groups interrupt the hydrophobic
self-association. Since ellagitannins are less hydrophobic than PGG (
8
,