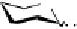Biology Reference
In-Depth Information
2.5
2.0
1.5
1.0
0.5
0.0
- 0.5
-1.0
-1.5
- 2.0
- 2.5
6
8
5
7
4
3
2
1
0
1
2
3
4
5
6
number of galloyl groups
Fig. 4.1 Partition coefficients of gallotannins.
1
: 1-
O
-galloyl-β-
D
-glucose (1);
2
: 1,6-di-
O
-galloyl- β-
D
-glucose (2);
3
: hamamelitannin (2);
4
: 1,2,6-tri-
O
-galloyl-β-
D
-glucose
(3);
5
: 1,2,3,6-tetra-
O
-galloyl-β-
D
-glucose (4);
6
: methyl 2,3,4,6-tetra-
O
-galloyl-β-
D
-
glucoside (4);
7
: 2,3,4,6-tetra-
O
-galloyl-β-
D
-glucose (4);
8
: 1,2,3,4,6-penta-
O
-galloyl-β-
D
-glucose (5). The number between parenthesis represents the number of galloyl groups
in the molecule.
R
1
β
-G
β
-G
β
-G
β
-G
β
-CH
3
H
β
-G
R
2
H
H
G
G
G
G
G
R
3
H
H
H
G
G
G
G
R
4
H
H
H
H
G
G
G
R
5
H
G
G
G
G
G
G
1
2
4
5
6
7
8
G
O
O
G
R
5
O
O
R
4
O
O
OH
OH
R
3
O
OR
1
O
OR
2
OH
HO
OH
G =
3
OH
4.2.2 Hydrophobic association of pentagalloylglucose and
ellagitannins with co-existing compounds
The most hydrophobic galloylated glucose, 1,2,3,4,6-pentagalloyl-β-
D
-
glucose (PGG,
8
), forms a gel in an aqueous solution at room
temperature (Fig. 4.3), indicating that the molecules are physically cross-
linked by weak hydrophobic and hydrogen bonding (Cai
et al.
, 1990).
The gel formation of
8
(PGG) was however disturbed by the presence of