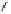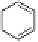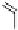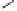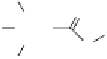Biology Reference
In-Depth Information
HO
OH
HO
OH
HO
HO
HO
H
H
O
S
HO
O
OH
OH
O
O
O
O
OH
HO
HO
OH
O
O
O
O
O
O
O
O
O
HO
HO
O
OH
OH
O
O
O
O
O
O
+ 1/2 O
2
OH
OH
HO
OH
OH
- H
2
O
HO
OH
OH
OH
HO
OH
HO
3
4
Fig. 3.10
In vitro
oxidation of 1,2,3,4,6-pentagalloylpyranose (
3
) to tellimagrandin II (
4
)
by a laccase-type phenolase from leaves of
Tellima grandiflora
(fringe cups). Oxidation
sites in the substrate are marked by a square box and a red arrow.
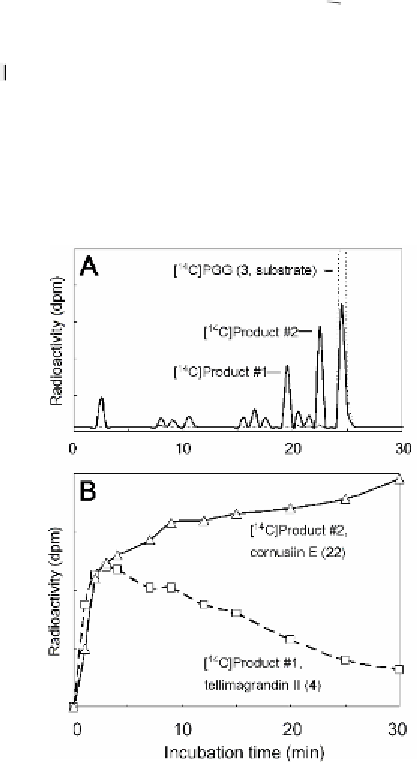
Fig. 3.11 Analysis and time course of reaction products of [
U
-
14
C]pentagalloylgluco-
pyranose (
3
) obtained using crude enzyme preparations from
Tellima grandiflora
leaves.
A
: RP-18 HPLC of
reaction products. (―) complete enzyme assay; (····), control with
acid-denatured enzyme.
B
: Time course of formation of reaction product #1
(tellimagrandin II,
4
; - -
- -) and its transformation to product #2 (cornusiin E,
22
; -Δ-).
Radioactivity was monitored by fractionation of eluates and liquid-scintillation counting.