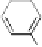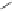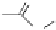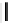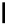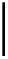Biology Reference
In-Depth Information
basis (Haddock
et al.
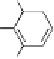
, 1982) which, as depicted in Fig. 3.2, can be
conveniently subdivided into several principal minor challenges, aiming
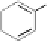
at (i) the biosynthetic route(s) to gallic acid (
1
) as the principal phenolic
unit; (ii) the origin of β-glucogallin (
2
) as the first specific intermediate
in the pathway to hydrolyzable tannins; (iii) the conversion of this
monoester to pentagalloylglucopyranose (PGG,
3
) along a series of so-
called “simple” galloylglucose esters; and finally the secondary
transformations of this pivotal intermediate to yield (iv) gallotannins by
adding galloyl
meta-
depsides groups (
7
), or to form (v) monomeric
ellagitannins by oxidative intramolecular aryl C-C coupling reactions,
followed by intermolecular C-O coupling leading to dimeric and
oligomeric derivatives that considerably contribute to the vast structural
diversity of this class of natural phenolic products.
COOH
OH
HO
OH
+Glc
O
HO
O
HO
HO
OH
OH
OH
OH
O
2
1
+ 4 Galloyl
OH
HO
HO
OH
HO
HO
HO
O
O
HO
OH
O
O
O
-n[H]
HO
OH
HO
O
O
O
O
O
HO
HO
Intramolecular
C-C coupling
OH
O
O
O
Monomeric
ellagitannins
with HHDP (5)-
residues
HO
OH
OH
OH
HO
OH
3
Intermolecular
C-O coupling
-n[H]
+ n Galloyl
Dimeric, oligomeric
ellagitannins
Gallotannins
Fig. 3.2 Principal steps in the biosynthesis of gallo- and ellagitannins. Glc =
glucopyranose.