Environmental Engineering Reference
In-Depth Information
particle interface. When it is dificult to fully distinguish among the mechanisms
of physical adsorption, chemical adsorption, and precipitation, the term
sorption
is
used to indicate the general transfer of material to the interfaces.
• Physical adsorption
: This occurs when the contaminants in the soil solution (aque-
ous phase, porewater) are attracted to the surfaces of the soil solids because of
the unsatisied charges of the soil particles. In the case of the heavy metals (metal
cations) for example, they are attracted to the negative charges exhibited by the
surfaces of the soil solids (Figure 2.12). When the cations are held primarily by
electrostatic forces, this is called
nonspeciic
cation adsorption.
• Speciic cation adsorption
: This refers to the situation where the ions penetrate the
coordination shell of the structural atom and are bonded by covalent bonds via O
and OH groups to the structural cations. The valence forces are of the type that
binds atoms to form chemical compounds of deinite shapes and energies. This
type of adsorption is also referred to as
chemisorption.
• Complexation with various ligands
:
Complexation
occurs when a metallic cation reacts
with an anion that functions as an inorganic ligand. Metallic ions that can be com-
plexed by inorganic ligands include the transitional metals and alkaline earth
metals. The inorganic ligands that will complex with the metallic ions include
most of the common anions, e.g., OH
−
, Cl
−
,
SO
2−
,
CO
2−
,
PO
3−
. Complexes formed
Side view
Anion
Metal cation
Specifically sorbed
(chemisorption)
Exaggerated isolated view
FIGURE 2.12
Exaggerated isolated view of interaction of metal cations (in the porewater) with soil particles. The soil particle
shown is a clay mineral particle.













































































































































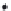









































































































































































































































































































































































































































































































































































Search WWH ::

Custom Search