Environmental Engineering Reference
In-Depth Information
500
Adsorption isotherm for kaolinite soil from
batch equilibrium tests
400
300
200
100
Equilibrium sorption characteristic
curve for kaolinite soil from column
leaching tests
0
0
5
10
15
20
25
Equilibrium concentration × 100 (ppm)
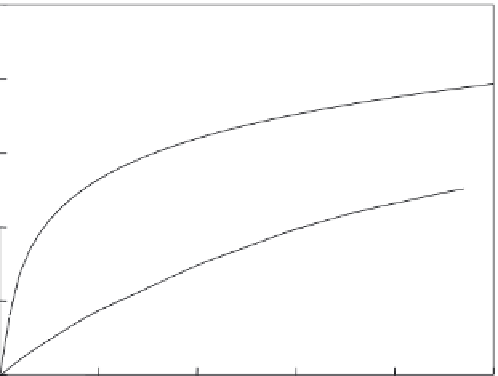
FIGURE 9.15
Comparison of Pb sorption curves obtained from batch equilibrium and column leaching tests for kaolinite soil.
(From Yong, R.N.,
Geoenvironmental Engineering: Contaminated Soils, Pollutant Fate and Mitigation
, CRC Press, Boca
Raton, 307 pp., 2001.)
event, it needs to be remembered that the
k
d
values would be upper limit values.
Competition for sorption sites, preferential sorption, and speciation-complexation
are some of the major factors that would directly affect the nature of the adsorp-
tion isotherm obtained.
For assessment of partitioning using soils in their natural compact state, it is necessary
to conduct column leaching or cell diffusion tests. In these kinds of tests, the natural soil
is used in the test cell or column, and either laboratory-prepared candidate contaminants
or natural leachates are used. The partition coeficient deduced from the test results is not
the distribution coeficient identiied with the adsorption isotherms obtained from batch
equilibrium tests. Instead, the partition coeficients obtained from column leaching or cell
diffusion tests need to be properly differentiated from the traditional
k
d
.
Yong (2001) has
suggested that these partition coeficients be called
sorption coeficients
to relect the sorp-
tion performance of the soils in their natural state in the column or cell. The disadvantages
in conducting column leaching and cell diffusion tests are (a) the greater amount of effort
required to conduct the tests, (b) the much greater length of time taken to obtain an entire
suite of results, and (c) inability to obtain exact replicate soil structures in the companion
columns or cells. The results indicate that the characteristic curves obtained from column
leaching tests, for example, are much lower than corresponding adsorption isotherms.
Figure 9.15 gives an example.
9.5.2 Organic Chemical Contaminants
The partitioning of organic chemical contaminants is a function of several kinds of inter-
acting mechanisms between the organic chemicals and the soil solids in the natural soil-
water system that constitutes the subsoil. A key factor in the development of the kinds














Search WWH ::

Custom Search