Environmental Engineering Reference
In-Depth Information
the rivers and 5% of the lakes in the United States. Groundwater quality is also impacted
with pesticide use.
Estimated mass balances of pesticides have been used to evaluate the fate and transport
of the pesticides in the environment, as illustrated in Figure 6.5. These estimates exhibit
substantial variation in some cases, due to, for example, atmospheric drift because of
weather conditions, application method, and properties of the pesticide. Unaccounted-for
pesticides can be due to the formation of covalent bonds with plant material or organic
matter of soils (Xu et al., 2003) or biodegradation.
The interactions of organic chemicals with soil organic matter (SOM) have been briely
discussed in Section 2.5 in Chapter 2 and is further discussed in detail in Chapter 9. These
discussions show that the association of pesticides with the organic matter of soil can be
described by the relationship:
C
oc
= k
oc
C
aq
,
where
C
oc
is the concentration of solute contami-
nant sorbed onto the soil organic carbon,
k
oc
is the organic carbon-water partition coefi-
cient, and
C
aq
is the dissolved concentration of the contaminant. The Freundlich adsorption
isotherm for many pesticides can be described by the following relationship
CkC
oc
n
=
1/
,
where
k
f
a nd 1/
n
are Freundlich parameters. These parameters are known for more than
60 pesticides (Barbash, 2005).
f
q
Atmosphere
3% lost to streams
30% associated with surface soil
and plant tissue
Aquifer
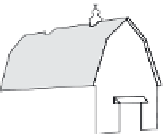
FIGURE 6.5
Estimates of the fate of pesticides after application to the soil for agricultural use (data from Barbash, 2005).
Percentages of pesticide associated with plants and other and lost to various to atmosphere, vadose zone, and
streams are dependent on highly climatic factors, soil management practice, type of pesticides used and man-
ner of application, etc.









































Search WWH ::

Custom Search