Environmental Engineering Reference
In-Depth Information
environmental problems when they are exposed to water and oxygen. Figure 5.5 gives
an illustrative example of what happens when pyrites (FeS
2
) are exposed to oxygen and a
source of water. Although oxygen and water are the two primary ingredients needed for
the development of the phenomenon commonly described as AMD, it must be noted that
microorganisms contribute signiicantly to the processes by way of catalyzing iron oxida-
tion, especially at pH levels below 3.5 (Manahan, 1990). The cycle of acid contact and oxida-
tion of the pyrite example shown in the diagram continues so long as oxidation processes
can proceed.
In the series of chemical reactions reported by Manahan (1990), beginning with the oxi-
dation of pyrite, the processes proceed as follows:
+
2
−
2
+
2
Fe
()+
s
2
HOO
+ →+
7
4
HSOFe
4
+
2
(5.1)
2
2
2
4
It is noted that because of the low pH levels, further iron oxidation of the pyrite can be
aided by various iron-oxidizing bacteria, as follows:
4Fe
2+
+ O
2
+ 4H
+
→ 4Fe
3+
+ 2H
2
O
(5.2)
3
+
2
+
2
−
+
FeSs
()+
14
Fe
+ →+
8
HO
15
Fe
2
SO
+
16
H
(5.3)
2
2
4
Sulfur oxidized to
sulfate
Release of ferrous ions
(Fe
2+
)
Pyrite, FeS
2
Exposure to oxygen
and water
Ferrous ions oxidized to ferric ions
(Fe
3+
)
Continued exposure in water results in
hydrolysis of ferrous ions and formation of
hydrated iron(III) hydroxide; Fe(OH)
3
Release of hydrogen ions
resulting in pH reduction
in fluid
Pyrite, FeS
2
Release of acidic iron and sulfate-rich fluid into the
geoenvironment and also back in contact with
exposed pyrite
Geoenvironment
FIGURE 5.5
Effect of exposure of pyrite to oxygen and water. Continued exposure to water will result in the generation of
iron hydroxide (yellowboy) and acidic solution that will be harmful to aquatic plants, animals, and will also
release heavy metals previously held by the soil. Similar reactions shown in the diagram will also occur for
sulides of copper, lead, arsenic, cadmium, and zinc.







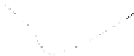













Search WWH ::

Custom Search