Environmental Engineering Reference
In-Depth Information
USEPA (1996), Test Methods for Evaluating Solid Waste, Physical/Chemical Methods, SW-846.
USEPA (1997), Manual for the Certification of Laboratories Analyzing Drinking Water: Criteria and
Procedures Quality Assurance, Office of Ground Water and Drinking, Cincinnati, OH. (http://
www.michgan.gov/deq).
USEPA (1998), Guidance for Data Quality Assessment: Practical Methods for Data Analysis. Report EPA/
600/R-96/084, Office of Research and Development, Washington, D.C. (http://www.epa.gov/quality/
qs-doc/g9-final.pdf).
USGS (1995), User's Manual for ANNIE, version 2, AComputer Program for Interactive Hydrologic Data
Management, USGS Water-Resources Investigations Report 95-4085, Reston, Virginia.(http://water.-
usgs.gov/software).
W
ILSON
N (1995), Soil Water and Groundwater Sampling, 1st Edition, CRC Press, Inc. Lewis Publishers,
Boca Raton, FL.
QUESTIONS AND PROBLEMS
1. Describe the difference between (a) accuracy and precision, and (b) method detection
limits and practical quantitation limits.
2. A water sample has 10 mg/L Hg
2þ
and a density of 1.0 g/mL (atomic weight of
Hg¼200.59). Calculate the following: (a) Hg
2þ
in ppb, (b) Hg
2þ
in mM, and (c) the
number of Hg
2þ
ion in 1 L of this sample containing 10 mg/L Hg
2þ
(Avogadro's
number¼6:02210
23
/mol).
3. The concentrations of arsenic (As) and selenium (Se) in a drinking water well were 2.0
and 3.8 ppb. (a) Convert arsenic concentration into ppm and mg/L, (b) Convert selenium
concentration into molarity (M) and micro-molarity (mM), (c) Have the concentrations
exceeded the maximum contaminant level (MCL) of 50 mg/L for both elements? The
atomic weight of Se is 79.
4. Carbon monoxide (CO) and hydrocarbons (HC) are the two main exhaust gases
produced by the combustion of gasoline-powered vehicles. Their concentrations are
regulated by required car inspections in some states. (a) Convert the regulatory standard
of 220 ppm HC into mg/m
3
(assuming a nominal molecular weight MW¼16). (b) If the
actual exhaust concentration of CO (MW¼28) at a low speed emission test is 0.16%,
what is the CO concentration in ppm and mg/m
3
?
5. The exhaust gas from an automobile contains 0.002% by volume of nitrogen dioxide
(MW¼46). What is the concentration of NO
2
in mg/m
3
at standard temperature and
atmosphere pressure (25
C and 1 atm pressure)?
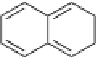
6. Indicate the category of the following organic compounds from this list: (1) aliphatic
acid, (2) aliphatic ether, (3) saturated aliphatic hydrocarbon, (4) polycyclic aromatic
hydrocarbon, (5) aliphatic alcohol, (6) aliphatic amine, (7) unsaturated aliphatic
hydrocarbon, (8) chlorinated hydrocarbon.
Cl
(a)
CH
3
CH
2
NH
2
H
3
C
(g)
(e)
(b)
CH
3
CH
2
COOH
CH
3
Cl
Cl
(c)
CH
3
CH
2
CH
2
OH
(h)
(f)
CH
2
CH
2
(d)
CH
3
CH
2
OCH
3



Search WWH ::

Custom Search