Environmental Engineering Reference
In-Depth Information
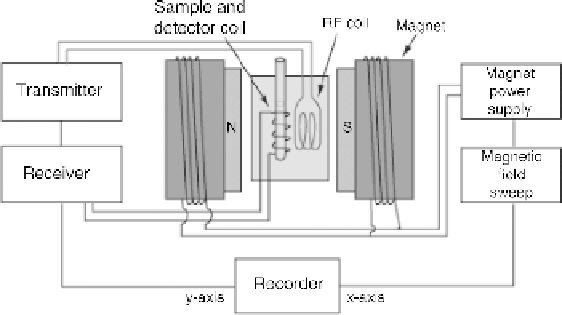
Figure 12.10
Diagram of a nuclear magnetic resonance (NMR) spectrometer (
#
Rod Nave,
HyperPhysics, 2006)
absorption of rf radiation, and a computer console as the terminal for controlling
each component and recording NMR spectra. The central superconducting magnet
system must be well maintained by regularly filling liquid nitrogen and helium
(typically every 10 days for N
2
and every 80-130 days for He). This is also the part
in which extreme safety procedures should be carefully followed to keep all
ferromagnetic items away from the magnet and avoid suffocation in a confined lab
space, where rapid boil-off of cryogenic liquid N
2
and He can occur. To acquire an
NMR spectrum, a small amount (mg) of the analyte is dissolved in 0.5 mL of solvent
(e.g., CDCl
3
or D
2
O) and the solution is placed in a long and thin-walled glass tube.
To understand the basic theory of NMR, we need to know the properties of nuclei,
the behavior of nuclei in a magnetic field, and the effect of a radio frequency radiation.
From elementary chemistry, we learned that a nucleus is made up of protons and
neutrons (positive and neutral charge, respectively). Circulating around the nucleus
are the negatively charged electrons having the same number as that of protons. For
example,
1
H nucleus has one proton and zero neutron in the nucleus and one electron
in the 1s orbital (Hence a
1
H nucleus is the same as a proton). Its isotope
2
H
(deuterium) has one proton and one neutron in the nucleus and one electron in the 1s
orbital. Similarly,
12
C has six protons and six neutrons in the nucleus and six electrons
around the nucleus (1s
2
2s
2
2p
2
; Refer to Chapter 8 for electronic configuration). Its
isotope
13
C has six protons and seven neutrons in the nucleus and six electrons.
From Pauli exclusion principle in elementary chemistry, we also learned that two
identical electrons in the same atom cannot have the same quantum number. These two
electrons must possess a property called spin, with spin quantum numbers (I)of
þ½and½. An electron in the nucleus is analogous to the Earth in relation to the Sun.
The Earth rotates once a year around the Sun and it spins along its own axis once a day.
Like electrons, nuclei also spin around its own axis at two spin states (quantum
numbers of þ½and½). In order for the nuclei to absorb electromagnetic radiation,
these two states must have different energies. One way to make them different is to
place the nuclei (sample) in a magnetic field. In the presence of a magnetic field (B
0
),
Search WWH ::

Custom Search