Environmental Engineering Reference
In-Depth Information
-
+
6
4
2
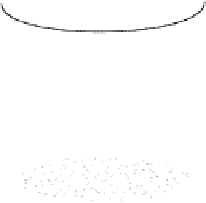
Figure 11.5
A membrane-based dissolved oxygen probe using
a polarographic cell [1¼Gold cathode where O
2
is reduced,
2¼Built-in ring-shaped reference electrode (Ag/AgCl), 3 ¼ O
2
permeable membrane (Teflon), 4¼Electrolyte solution (KCl),
5¼O-ring to hold replaceable membrane, 6¼Insulating rod]
1
5
O
2
O
2
3
Ag anode surrounded by a gold (Au) cathode. The commonly known Clark oxygen
sensor patented by L. C. Clark in 1956, as shown in Figure 11.5, is the polarographic
type. Galvanic probes are more stable and more accurate at low DO levels than
polarographic probes. Galvanic probes often operate for several months without
electrolyte or membrane replacement, whereas polarographic probes need to be
recharged more frequently.
Despite the structural difference between the two, there are similarities in the
operational principles. In both types, dissolved oxygen diffuses across a
permeable membrane and is reduced in a reaction with a fill solution generating
a current flow from cathode to anode. This current flow is proportional to the
amount of oxygen that crosses the membrane. Using the Clark-type cell as an
example, upon oxygen permeation through the membrane, oxygen is reduced at
the gold cathode and the coupled oxidation reaction occurs at the silver reference
electrode (anode):
O
2
þ2H
2
Oþ4e
!
4OH
ðcathode reactionÞ ð11
:
13Þ
4AgðsÞþ4Cl
!
4AgClðsÞþ4e
ðanode reactionÞ
ð11
:
14Þ
In practical uses, one should handle dissolved oxygen probes with extreme care.
Inaccurate or even erroneous readings may be caused by a loose, wrinkled, or fouled
membrane. The probe should be calibrated on a frequent basis. The calibration can
be done by one of the three means, that is, the use of air with 100% relative humidity,
air-saturated water prepared by aeration, or by the wet chemical method (Winkler
method) introduced in Chapter 6.
EXAMPLE 11.1.
The coupled redox reactions shown in Eqs 11.13 and 11.14 have standard
potentials of þ0:40 and 0:22 V, respectively. (a) Develop an overall balanced reaction




Search WWH ::

Custom Search