Environmental Engineering Reference
In-Depth Information
instrument with mass spectrometers and other instruments requiring small
solvent input volumes. The narrow bore column can be used for a flow of a
few
L/min such as those used in LC/MS. Since most of the environmental
organic contaminants are hydrophobic (i.e., solvent soluble compounds), a
reverse phase C
8
or C
18
column is recommended. C
8
is an excellent starting
point for method development because of its moderate hydrophobicity.
m
10.1.4 Terms and Theories of Chromatogram
A chromatogram is the instrumental output of all chromatographic analyses. It is a
plot of detector signal vs. time after sample introduction, that is, the time from
sample injection to the time when detector responds to the compound (Fig. 10.2).
The chromatograms are the same regardless of GC, HPLC, or any other
chromatographic methods. They vary only with the types of signals. In this section,
we define several important terms regarding chromatograms and introduce several
quantitative equations helpful to further understand chromatographic separation.
Detailed mathematical derivations are avoided to simplify the illustration.
t
rB
t
r
t
rA
t
m
Baseline
unretained peak
W
B
W
A
0
Time
Figure 10.2
A typical chromatogram showing an un-retained peak and two analyte peaks
Shown in Figure 10.2 are a peak of an unretained compound at time t ¼ t
m
, the first
eluted compound (A) at t ¼ t
rA
, and the second eluted compound (B) at t ¼ t
rB
.Here,
t
m
is the mobile-phase holdup time, defined as the time required for an average molecule
of the mobile phase to pass through the column; t
r
is the retention time, corresponding to
the time it takes after sample injection for the analyte to reach the detector.
The peak of an unretained compound may be due to the response to air or
methanol in GC (air peak) or the solvent in HPLC (solvent peak), which may not be
present at all times, depending on whether or not the detector has a response to such.
Another feature of chromatogram is the broadening of peaks as the time increases.
This broadening is typical in any chromatography because of the random diffusion
processes in the column. This is to say that various molecules of the same compound
elute from the column at slightly varied times. A third feature of a typical
chromatogram is the symmetrical nature of all eluting peaks. The symmetry can be
mathematically described by the Normal (Gaussian) distribution introduced in
Chapter 2.
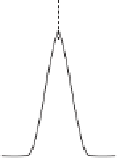


















Search WWH ::

Custom Search