Environmental Engineering Reference
In-Depth Information
Flame
or furnace
Monochromator
P
o
P
+
Light source
Chopper
Detector
Readout
Sample
Figure 9.3
Instrument components for a single-beam atomic absorption spectrometer
to be familiar with the light source and the atomizer rather than the optical and electrical
detection components. The latter can be effectively considered a ''black box'' since their
repair and maintenance are generally beyond the ability of most analytical chemists.
1. Light source: The light source is usually a hollow cathode lamp (HCL) of
the element being measured. Its purpose is to provide the spectral line for
the element of interest. The HCL uses a cathode made of the element of
interest with a low internal pressure of an inert gas. A low electrical current
(10 mA) is imposed in such a way that the metal is excited and emits a few
spectral lines characteristic of that element (For instance, Cu has 324.7 nm
and a couple of other lines; Se has 196.0 nm and other lines.) The light is
emitted directionally through the lamp's window, which is made of a glass
transparent in the UV and visible wavelengths.
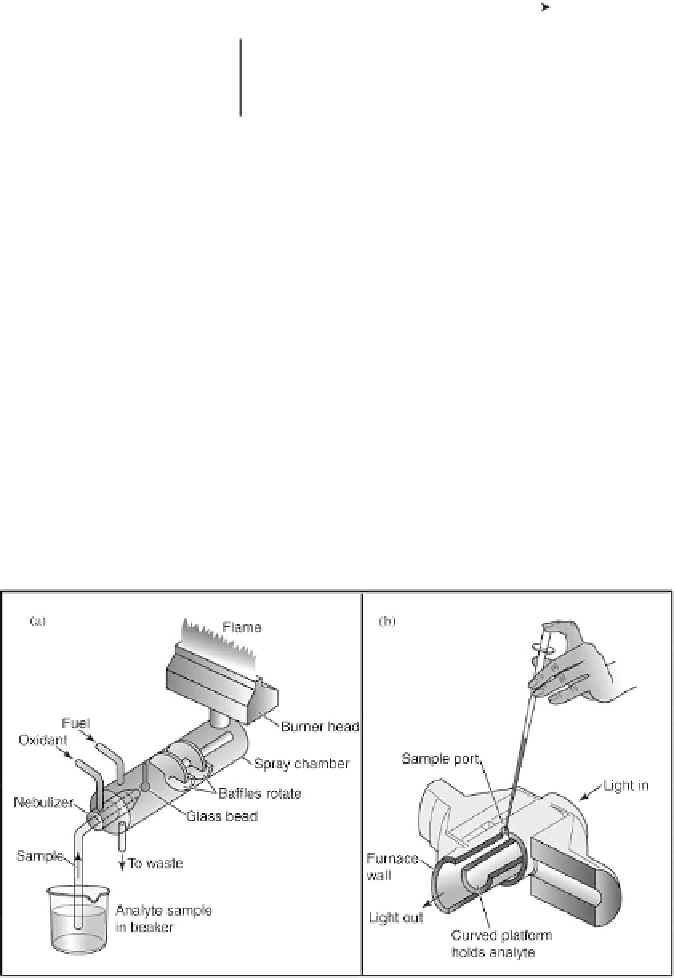
2. Nebulizer and atomizer: In a flame system (Figure 9.4a), the nebulizer is
to suck up liquid sample at a controlled rate, create a fine aerosol, and mix
Figure 9.4
Diagram of (a) a premix nebulizer burner for a flame atomic absorption spectrometer,
and (b) a graphite furnace for a flameless atomic absorption spectrometer (Girard, JE, Principles of
Environmental chemistry, 2005, Jones and Bartlett publishers, Sudbury, MA.
WWW.jbpub.com
.
Reprinted with permission.)











Search WWH ::

Custom Search