Environmental Engineering Reference
In-Depth Information
Chemicals can absorb electromagnetic radiation, but only at certain energies
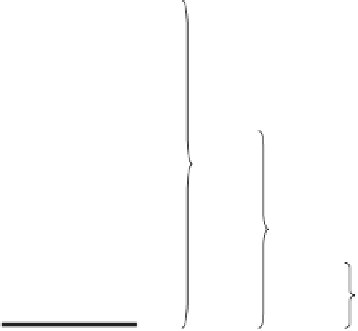
(wavelengths). Figure 8.2 illustrates the relationships between different energy
levels within a molecule. The three groups of lines correspond to different electronic
configurations. The lowest energy, termed the ground state
, is the most stable
electron configuration. Photons having certain energies in the visible and UV
regions of the spectrum can cause these electrons to be excited into higher
energy orbitals. Some of the possible absorption transitions are indicated by the
vertical arrows. Photons that are more energetic (UV to X-ray region) may
cause an electron to be ejected from the molecule (
ionization
). Photons in the
infrared (IR)
region of the spectrum have much less energy than those in the
visible or UV regions of the electromagnetic spectrum. They can, however,
excite vibrations in molecules. There are many possible vibrational levels within
each electronic state. Transitions between the vibrational levels are indicated by
the vertical arrows on the left side of the diagram.
E
0
= Ground electronic states
E
2
E
1
= First electronic excited states
E
2
= Second electronic excited states
UV
E
1
VIS
IR
E
0
Figure 8.2
Energy levels in a molecule and three types of absorption spectrometry. E
is the radiation absorbed by a molecule
Figure 8.3 further illustrates molecular responses to radiation including UV, VIS,
IR, and microwave. As can be seen, electron transition or ionization can occur under
high energy UV radiation. The least energetic microwave radiation
cannot excite
vibrations but can only cause molecules to
rotate
. Microwave ovens are tuned to
the frequency that causes molecules of water to rotate, causing heat as a result of
friction of water-containing substances (Eubanks et al., 2006).
The absorption
described in Figures 8.2 and 8.3 is only one type of the
electromagnetic radiation. Absorption of radiation moves the atom to a higher
energy level. Transitions among the vibrational and rotational states give rise to
absorption at IR wavelengths, whereas those between electronic levels involve
more energetic visible or UV radiation.
On the contrary, the energy at the higher state may also return to ground state by
emission
or may lose some of its energy as thermal energy and return to the



Search WWH ::

Custom Search