Environmental Engineering Reference
In-Depth Information
100
Biomass carbon
100%
Energy
Production
Biochar
Biochar carbon
100%
50
Biomass carbon
100%
Uncharred organic matter
100 years
10
Biomass carbon
0%
Biochar carbon
>40%
1 2 3 4 5
Ye a r s
(a)
(b)
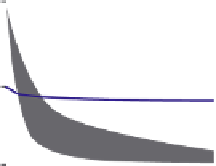
Figure 11.3.1
Biochar: energy and decomposition
Time scale for biomass and bio-char sequestration after charring and decomposition in
soil.
Figure adapted from Lehmann et al.
[11.8].
occurs faster, causing the CO
2
levels to decrease. The time scale of this
process is several orders of magnitude slower than our rate of emission.
If we could accelerate the natural weathering process, we would be able
to reduce CO
2
levels.
The idea is to add abundant minerals (e.g., olivine,
Box 11.3.2
) to
soil used for agriculture [11.9]. The scale of the operation of course
would have to be enormous. One would need to mine, grind, transport,
and spread these rocks over fi elds. The volume of olivine needed would
be on the order of 7 km
3
per year, which is about twice the amount of
coal we mine. At present, little is known on the potential impacts of
these weathering reactions on the soil. Alternative proposals involve
conducting the weathering reaction in a chemical engineering plant
and then releasing the resulting bicarbonate solutions into the sea.
An advantage of these methods is that all chemicals are already
present in large quantities in the soils and the oceans. Of course, one
has to mitigate the effects of the large concentrations. For every CO
2
molecule sequestered, one needs a mineral molecule. As a conse-
quence, the amount of material we need will be enormous, most likely
exceeding in mass the amount of CO
2
we need to sequester. Mining
such enormous quantities of material would have signifi cant impacts on
the environment, would be expensive, and would create ancillary energy
(and thus carbon) costs.











Search WWH ::

Custom Search