Environmental Engineering Reference
In-Depth Information
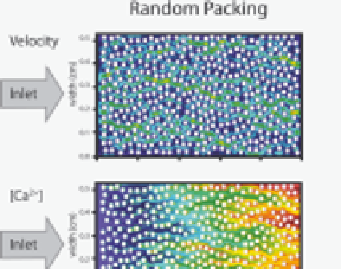
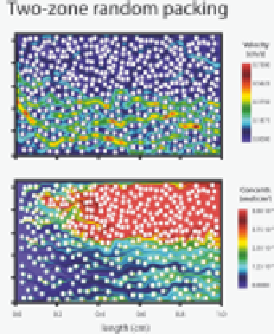
Figure 9.8.9
Reactive transport simulation of CO
2
-acidifi ed water fl owing through a
bed of calcite beads
Two-dimensional adaptive mesh refi nement simulation of CO
2
-rich water fl owing
through a bed of calcite beads. The fi gures on the left side show the case where the
beads are randomly distributed; the right side shows the case where the beads are more
densely distributed in one half of the simulated system. Upper and lower fi gures show
the velocity fi eld and Ca
2
+
concentrations, respectively. The inlet water is at pH 5 with
p
CO
2
=
3.15
×
10
−
4
bar, 0.01 M NaCl, and 0.1 cm s
−
1
velocity. The calculations predict
that the average (upscaled) dissolution rate of calcite in the heterogeneously-packed
system (right) is lower than in the homogeneously-packed system (left), i.e., heterogene-
ity can be viewed as reducing the “effective” reactive surface area.
Figure reproduced
from Molins et al.
[9.42],
with permission from John Wiley and Sons.
reaction in porous media. The demonstration projects on sequestration
(see Chapter 8), however, have been successful both as pilot models for
large-scale storage and as valuable sources of data that can be used to
test models. The success of the existing demonstration projects shows
that geological storage of CO
2
is a technology that is already applicable
at the industrial scale. Opportunities exist for improving existing models
and for signifi cantly optimizing the effi ciency of CO
2
storage operations.












Search WWH ::

Custom Search