Environmental Engineering Reference
In-Depth Information
Solubility of CO
2
in groundwater
The solubility of CO
2
in water is another key physical property that has
important consequences for controlling solubility trapping (see Chapter 8).
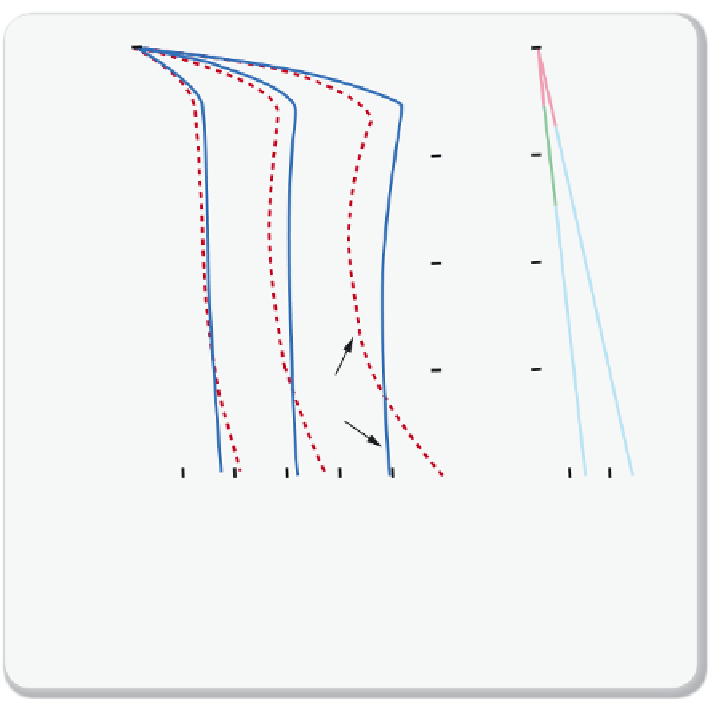
Figure 9.3.3
shows the solubility profi les for CO
2
in groundwater as a
function of depth or pressure for 0, 2, and 6 molar NaCl brine at two dif-
ferent
P
-
T
conditions. The fi gure shows that the solubility of CO
2
increases rapidly with depth, and then declines slightly as both pressure
and temperature increase and CO
2
becomes supercritical.
0
Pure water (0 m NaCl)
Gas
Liquid
1000
100
Brine (2 m NaCl)
2000
200
Supercritical
Hypersaline brine
(6 m NaCl)
3000
300
30ºC/km
15ºC/km
4000
0 0.005 0.01 0.015 0.02 0.025 0 50 100
T (ºC)
x
li
CO
2
Figure 9.3.3
Solubility of CO
2
in fresh and saline water as a function of depth
Solubility of CO
2
in fresh and saline water as a function of depth or pressure at varying
P
-
T
conditions (left) and temperature profi les that highlight the various phases that CO
2
assumes (right). The red dashed lines are for a temperature profi le of 30
°
C/km and the
blue solid lines for a profi le of 15
°
C/km.
Figure adapted from Oldenburg
[9.11]
.








Search WWH ::

Custom Search