Environmental Engineering Reference
In-Depth Information
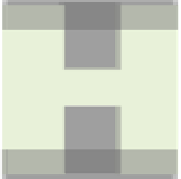
Figure 7.6.1
Microscopic model of a membrane
A small part of a simple microscopic model of a nanoporous membrane; the green area
is accessible to the molecules. The membrane channels consists of cavities (of size
L
cy
×
L
cz
) that are separated by windows (of diameter
L
wy
). In the cavities the energy of the
molecule is
U
c
and in the windows
U
w
. The dark blue shading indicates membrane mol-
ecules and we assume gas molecules do not occupy that space.
self-diffusion coeffi cient is a reasonable approximation of the Fick diffu-
sion coeffi cient. At this point, we emphasize that at real fl ue gas condi-
tions these assumptions may not hold true for all materials.
We introduced this model in order to analytically compute simple
expressions for both the Henry coeffi cient and the self-diffusion coeffi -
cient for a simple (spherical) gas molecule. The formulae we derive allow
us to compute the permeation and permeation selectivity. Hence, this
model allows us to develop some intuition about what a Robeson plot
would look like for our nanoporous materials. However, by now you must
have developed a healthy skepticism about our intuition regarding mem-
brane behavior!
Adsorption
We saw in the adsorption section that we can compute the Henry coef-
fi cient of adsorbed molecules by randomly inserting a molecule and
computing its average energy:
1
−
Uk T
/
H
=
〈
e
〉
B
random
kT
B





























Search WWH ::

Custom Search