Environmental Engineering Reference
In-Depth Information
p
R
ρ
p
P
ρ
ρ
(g)
R
ρ
(g)
P
z
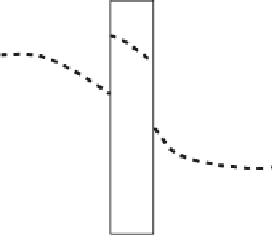
Figure 7.2.2
Concentration profi le
The concentration profi le of a pure component across a membrane; the dotted lines are
the real profi les, while the solid lines assume that we have perfect mixing on the two
sides of the membrane. The concentration
(in moles per unit volume) has a superscript
“(g)” if it is in the gas phase. The pressure on the retentate and permeate sides are given
by
p
R
and
p
P
, respectively.
ρ
j
(
z
)
j
(
z
+
dz
)
ρ
(
z
)
ρ
(
z
+
dz
)
z
+
dz
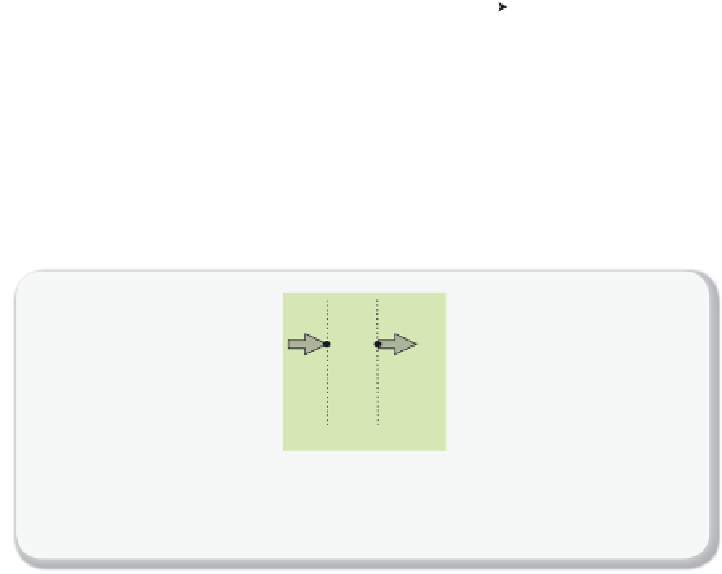
Figure 7.2.3
Mass Balance
Mass balance over part of the membrane:
ρ
(
z
) is the concentration (mol/volume) of the
gas molecules across the membrane and
j
(
z
)
is the fl ux [mol/(sec unit area)].
If we use Fick's equation in the expression for the mass balance, we get
the following differential equation for the time-dependent concentration
profi le:
2
d
ρ
d
ρ
=
D
2
dt
dz
If we assume that we have a steady state, such that the time derivative
is equal to zero, the concentration depends only on the position and we
















Search WWH ::

Custom Search