Environmental Engineering Reference
In-Depth Information
above a plate. One should therefore view a design based on equilibrium
data only as the ideal reference. In a real design, we have to correct for
the fact the system is not actually in equilibrium. These corrections always
make the design bigger and consequently more expensive.
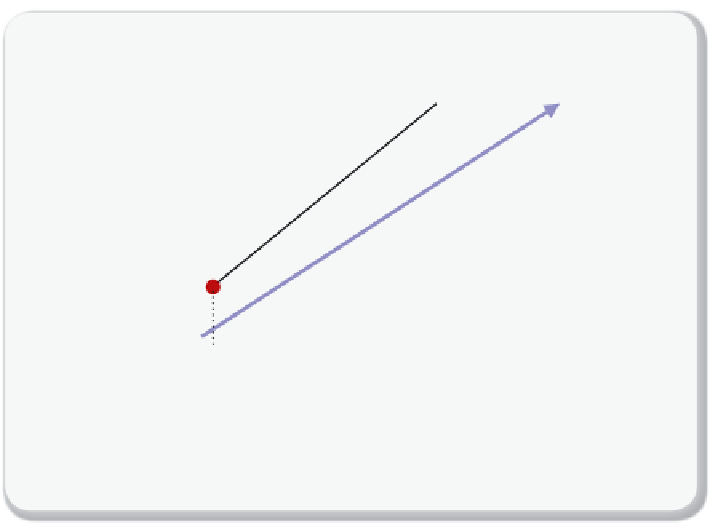
Figure 5.4.1
illustrates this point. For a given gas composition, the corresponding liq-
uid concentration of CO
2
will be lower compared to the true equilibrium
concentration. As a result, a greater number of stages is required.
Next, we will try to quantify the two main processes that preclude
equilibrium in the froth.
Diffusion limitation
If we have thermodynamic equilibrium, the concentrations of CO
2
in the
gas and liquid phase are in equilibrium. If we know the equilibrium con-
centration, we can compute the fl ow we need to remove all the CO
2
that
is coming from the fl ue gas. In this calculation it is assumed that equilib-
rium is reached instantaneously. In reality CO
2
molecules have to diffuse
from the gas phase into the liquid phase.
Figure 5.4.2
shows an
y
CO
2
flue
y
CO
2
#1
#2
#3
exh
y
CO
2
#4
reg
x
CO
2
x
CO
2
Figure 5.4.1
Number of plates for non-equilibrium
Number of plates for a system that does not reach the (blue) equilibrium
concentration.










Search WWH ::

Custom Search