Environmental Engineering Reference
In-Depth Information
salt. As a result, these “frustrated” salts remain liquid at room tempera-
tures. Like molten salts, they have special properties because of their
ionic character. For example, ILs have relatively low vapor pressures
compared to ordinary liquids. Because of charge neutrality, IL molecules
can only escape in pairs from the liquid phase. As we will see in one of
the next chapters, this makes it possible to use ILs as membrane
materials.
Another interesting property of ionic liquids is that they are highly
customizable. By changing the cation and anion, it is possible to form
millions of different ionic liquids, many of which chemists have already
R
2
O
O
N
-
N
N
F
S
S
F
R
1
N
+
O
O
F
F
F
F
R
3
bis[(trifluoromethyl)
sulfonyl]amide
(a)
0.20
0.18
N
+
N
+
N
+
N
+
N
+
N
+
N
+
N
N
N
N
N
N
N
N
N
N
N
N
N
N
0.16
Si
O
O
N
0.14
O
O
0.12
O
O
0.10
0.08
0.06
0.04
0.02
(b)
Figure 5.3.5
Triazolium-based ionic liquids
(a) Example of a triazolium-based ionic liquid. The bulky cations and anions make it very
diffi cult for these liquids to crystalize and hence at conditions where ordinary ionic
systems (salts) are solid, these materials are liquid. By varying the groups R
1
, R
2
, and
R
3
one can make millions of different materials each having very different properties.
(b) Effect of the structure of the ionic liquid on the solubility of CO
2
.
Figure based on
data from
[5.10].














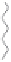





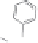



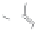

































Search WWH ::

Custom Search