Environmental Engineering Reference
In-Depth Information
of the operating line (increasing
n
sol
/
n
fl ue
, see
Figure 5.2.8
) or to change
the properties of the solvent, in order to lower
κ
, and hence the slope of
the equilibrium line.
As shown in
Figure 5.3.1
, an increase in
κ
increases the number of
plates; a decrease in
is defi ned as the Henry constant divided by the total pressure. The Henry
constant depends on the solute, the solvent, and the temperature. One
way to reduce the slope of the equilibrium line would be to raise the total
pressure of the absorber, but as noted in Chapter 4, compressing large
volumes of gasses takes signifi cant amounts of energy. The natural alter-
native to altering pressure would be to somehow lower Henry's constant.
We either have to change the solvent or add something that can help
water take up CO
2
more effi ciently. There are currently quite a few
research opportunities in CCS that involve designing molecules to facili-
tate the uptake of CO
2
.
κ
makes the absorber more effi cient. Recall that
κ
Water and water+
Up until this point in the chapter, we have assumed that the absorption
of CO
2
in water is a simple equilibrium between CO
2
in the gas phase and
in solution. In reality, the situation is more complex. When added to
water, CO
2
forms multiple ions, dissociating to become carbonic acid
(H
2
CO
3
), bicarbonate (HCO
3
−
), and carbonate (CO
3
2
−
). To understand the
y
CO
2
y
CO
2
flue
flue
y
CO
2
y
CO
2
#1
#2
#2
#3
exh
y
CO
2
y
CO
2
exh
#1
# 4
reg
x
CO
2
x
CO
2
reg
x
CO
2
x
CO
2
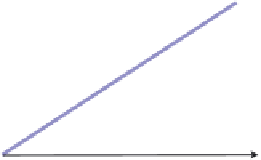
Figure 5.3.1
Effect of the solvent on the number of plates
McCabe-Thiele diagrams for different solvents: the left fi gure has a solvent with a higher
κ
and the right fi gure a solvent with a lower
κ
.







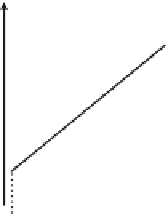











Search WWH ::

Custom Search