Environmental Engineering Reference
In-Depth Information
This equation is the fi rst of two equations that we can use to graphi-
cally describe the absorption process in a plate tower such as that shown
in
Figure 5.2.1
. In particular, if we assume the fl ue gas and the solvent
are at thermodynamic equilibrium, we can use this equation to relate the
mole fractions of CO
2
in the liquid and the gas on each of the plates. This
function is called the equilibrium line and is shown schematically in
Figure 5.2.3
. As we vary the design components of our multi-plate
absorption column, we can assume that the equilibrium of the frothy
liquid/gas mixture behind the weir can be described by a point on this
line. The slope of the equilibrium line,
, represents the physical proper-
ties of our solvent, and this simple constant gives us suffi cient informa-
tion on the thermodynamics of CO
2
absorption to design our separation.
Of course, Henry's law only approximates the solubility. Comparison with
experimental data shows that Henry's law only holds for very low con-
centrations of CO
2
in the solvent. A more accurate design would require
taking this non-ideal behavior into account (and this is exactly what the
more sophisticated design tools will do for you).
κ
Conservation of mass
Now that we have described the equilibrium thermodynamics of our
absorber, we can integrate another relationship into this system: conser-
vation of mass. As we mentioned above, the amount of CO
2
that goes
into the system must be equal to the amount of CO
2
that comes out of
Figure 5.2.3
The equilibrium line




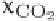




Search WWH ::

Custom Search