Environmental Engineering Reference
In-Depth Information
the exterior of catalyst particles. Pore volume and size distribution of the catalyst may play a
key role in determining this ratio.
Apparently, before separation from porhyrins, V and Ni may coordinate with sulfur of the
active metal sulfide. Potential coordination with the active phase such as Co(Ni)-Mo(W)-S
could lead to the change in activity of the active sites. In this regard, V is expected to have
more detrimental effect than Ni. Thus, its interaction may lead to the formation of the V-Mo-S
phase which is less active than the Co(Ni)-Mo(W)-S phase.
With progressive growth of the metal deposits, the pore diameter becomes less than the
molecular diameter of porphyrin molecules. This prevents the access of the reactant molecules
to the interior. At this stage, an abrupt loss of the catalyst activity is usually observed
[264]
.
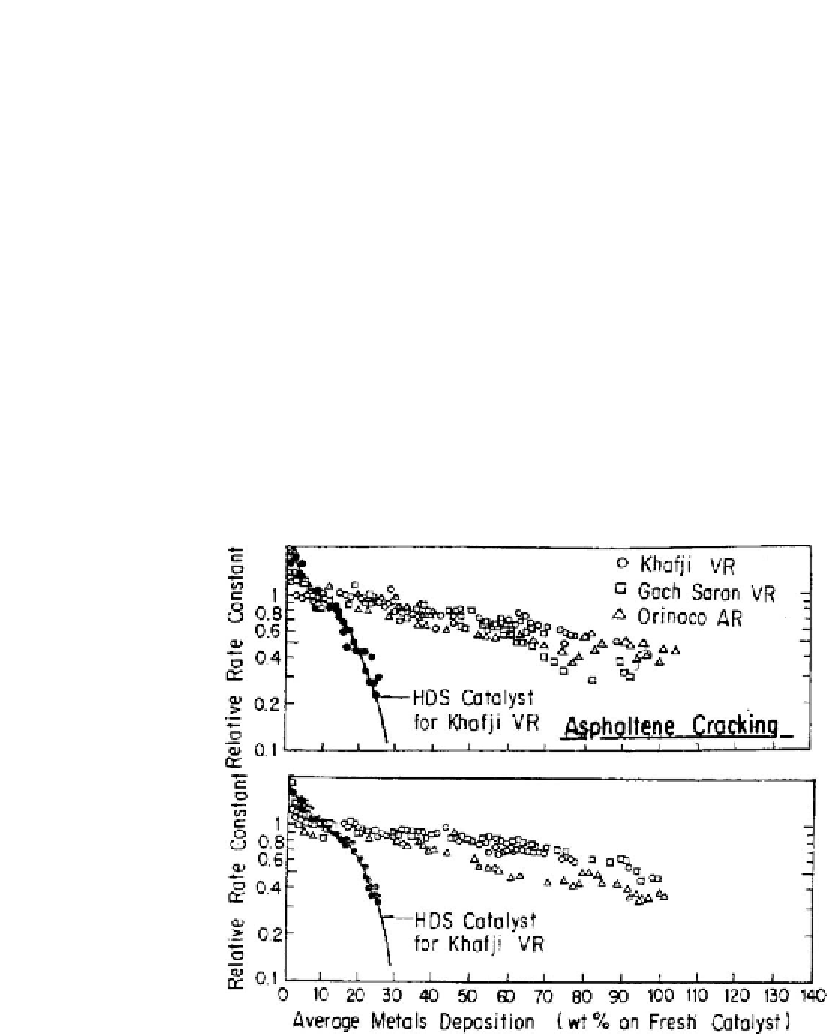
However, to a great extent, this point depends on the metal retention and/or metal storage
capacity of the catalyst, which in turn is influenced by catalyst porosity. For example, for
typical HDS catalyst, metal retention before almost total deactivation, may approach 20 wt.%
or even less as it is indicated in
Fig. 4.23 [265,266]
. Although the sudden decline in HDS
activity of HDM catalyst was observed at 50% metal retention, its activity for HDM and HDAs
was still retained suggesting that the deposition of metals could continue beyond this point. It
Figure 4.23: Effect of metal deposition on asphaltenes conversion and vanadium removal for
hydrodesulfurization (HDS) and hydrodemetallization (HDM) catalysts [From refs
265 and 266
.
Reprinted with permission].