Environmental Engineering Reference
In-Depth Information
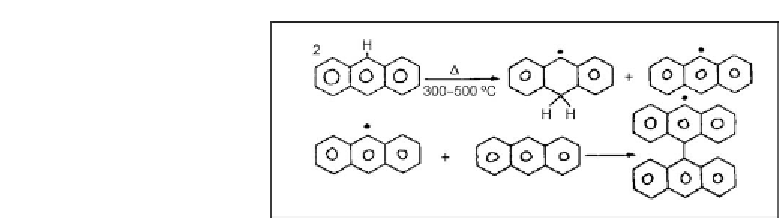
Figure 4.19: Free radical mechanism for formation of coke from anthracene [From ref.
219
.
Reprinted with permission].
of the free radical mechanism. Theoretically, the active surface hydrogen in the form of SH
and MeH entities may stabilize radicals as well. However, at later stages on stream, this radical
scavenging source may be exhausted due to the diminished hydrogen activation caused by the
extensive catalyst deactivation. This is supported by the observations made in commercial
units, i.e., a rapid coke build-up during final stages on stream.
The involvement of carbocations during coke formation is also possible. Carbocations are the
important intermediates of some reactions, i.e., hydroisomerization (HIS), hydrocracking
(HCR), polymerization, etc. If not stabilized, carbocations can combine to higher molecular
weight species. The coupling of polynuclear aromatics leading to coke precursors and finally
to coke was also proposed
[224-230]
. The rate of such reactions was enhanced in the presence
of the Bronsted acid sites. This indicates the involvement of proton (via carbocation) during
coke formation. The coke formation was significantly diminished after Bronsted acidity was
destroyed by pretreating the catalyst support with basic species. Carbocation mechanism may
be part of the overall mechanism of coke formation regardless the origin of the heavy feed. In
the case of such mechanism, the type of the support may be more important factor than the
type of the feed. It has been generally observed that the rate of some hydrocarbon reactions
(cracking, isomerization, polymerization, etc.) was rather low unless the source of protons was
available. In this regard, the catalysts supported on acidic supports (e.g., zeolites) are most
suitable.
With respect to the acidity of support, carbon may represent another extreme to zeolites. Thus,
it is unlikely that acidic sites are present unless carbons were subjected to special
pretreatments. However, C
H bonds may be present because of hydrogen activation on carbon
can proceed
[231]
. The results on hydroprocessing of the Kuwait AR conducted by Nakamura
et al.
[223]
over the carbon-supported catalysts were interpreted in terms of the free radicals
mechanism. In the case of the Co(Ni)/Mo(W) catalysts supported on carbon, the SH groups
could be a source of the hydrogen necessary for quenching radicals unless the heavy feed
involved was of a naphthenic origin. Under certain conditions, SH groups may possess a
Bronsted acid character. For example, the Bronsted acid character of such groups increased