Environmental Engineering Reference
In-Depth Information
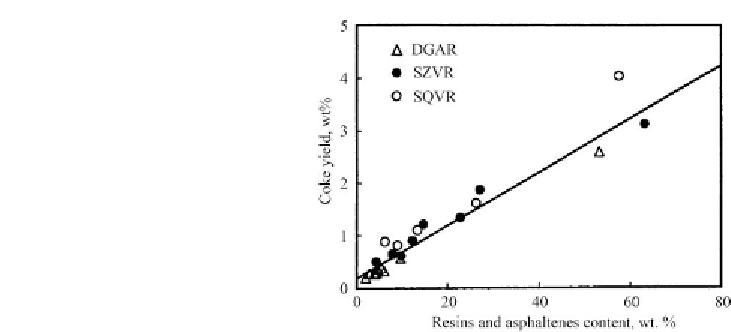
Figure 4.10: Coke on catalyst as function of the content of resins and asphaltenes in fractions
from Dagang AR (DGAR), Saudi light VR (SQVR) and Saudi medium VR (SZVR) at 673 K and
8.5MPa of H
2
over NiMo/Al
2
O
3
[From ref.
191
. Reprinted with permission].
unlikely that these observations can be generally applied to all heavy feeds because the
chemical structure of asphaltenes may be another parameter influencing coke deposition.
Thus, for heavy feeds having a similar content of asphaltenes, but of different chemical
structure, the coking propensity increased with the increasing aromaticity of asphaltenes.
It is believed that during very early stages of the operation, there is little effect of metals on
coke formation. On the other hand, the coke formed initially can have a pronounced effect on
the rate of the metal deposit formation because of the partial pore plugging by coke. Moreover,
this part of the support on which metals could deposit was already occupied by coke. It is
therefore critical that the rate of coke formation is kept at minimum to ensure a high HDM
activity of catalysts. In this regard, the results in
Fig. 4.4 [174,175]
can have important
implications on the design and preparation of the HDM catalysts, although they were obtained
for a VGO feed. Thus, the coke formation may be kept at a minimum by selecting an optimal
composition of catalyst. At the optimal composition, formation of the “chemical coke”
associated with hydroprocessing reactions is slow, thus ensuring a high HDM activity due to
the diminished interference by coke. However, this was not confirmed in the study involving
the Kuwait atmospheric residue (90 ppm of V+Ni; 3.6 wt.% asphaltenes) conducted by Marafi
et al.
[193]
who compared the Mo/Al
2
O
3
(3 wt.% Mo) with NiMo/Al
2
O
3
(8 wt.% Mo and
2 wt.% Ni) catalysts having pore volume of 0.7 and 0.5mL/g, respectively. Typically, the
catalysts were used for HDM and HDS, respectively. Between 633 and 693 K and at 12MPa,
consistently more coke was deposited on the HDM catalyst. As expected, the H/C ratio of coke
on the HDM catalyst was much lower than that on the HDS catalyst because of the higher
HYD activity of the latter. The contradictory results reported in the literature underline
complexity of the simultaneous deactivation of catalyst by coke and metals, particularly during
the initial stages. This may be attributed to the differences in experimental conditions. This is