Environmental Engineering Reference
In-Depth Information
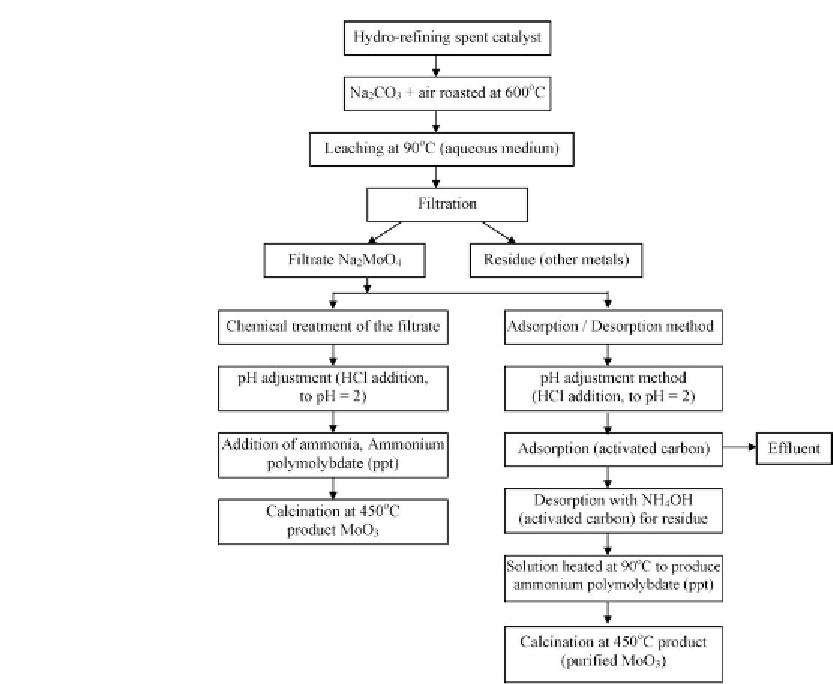
Figure 11.15: Schematic diagram for the production of high purity molybdenum oxide from
hydrorefining spent catalyst [From ref.
657
. Reprinted with permission].
(
Fig. 11.17
)
[567]
, spent catalysts are mixed with alkaline materials, such as sodium carbonate
and roasted at temperatures between 1400 and 1800
◦
F (650-900
◦
C), to convert molybdenum,
vanadium, and part of the sulfur into their respective soluble sodium salts. The roasted
material is leached with water to obtain a solution laden with molybdenum and vanadium, and
a residue containing a portion of the molybdenum and vanadium, all the alumina, nickel,
cobalt, silica, and some sodium in the form of sodium aluminum silicate. The solution is
treated with ammonium salts to separate vanadium metavanadate, which may be converted
into vanadium pentoxide. The remaining solution is further acidified to precipitate molybdic
acid, which may be converted into molybdic trioxide. The leach residue is dried and reduced in
the presence of carbon to produce a high grade alloy of nickel and cobalt, fused alumina,
substantially free of sodium, and to vaporize most of the sodium. The sodium is recovered in
the form of sodium hydroxide and is recycled to the roasting and leaching operations.