Environmental Engineering Reference
In-Depth Information
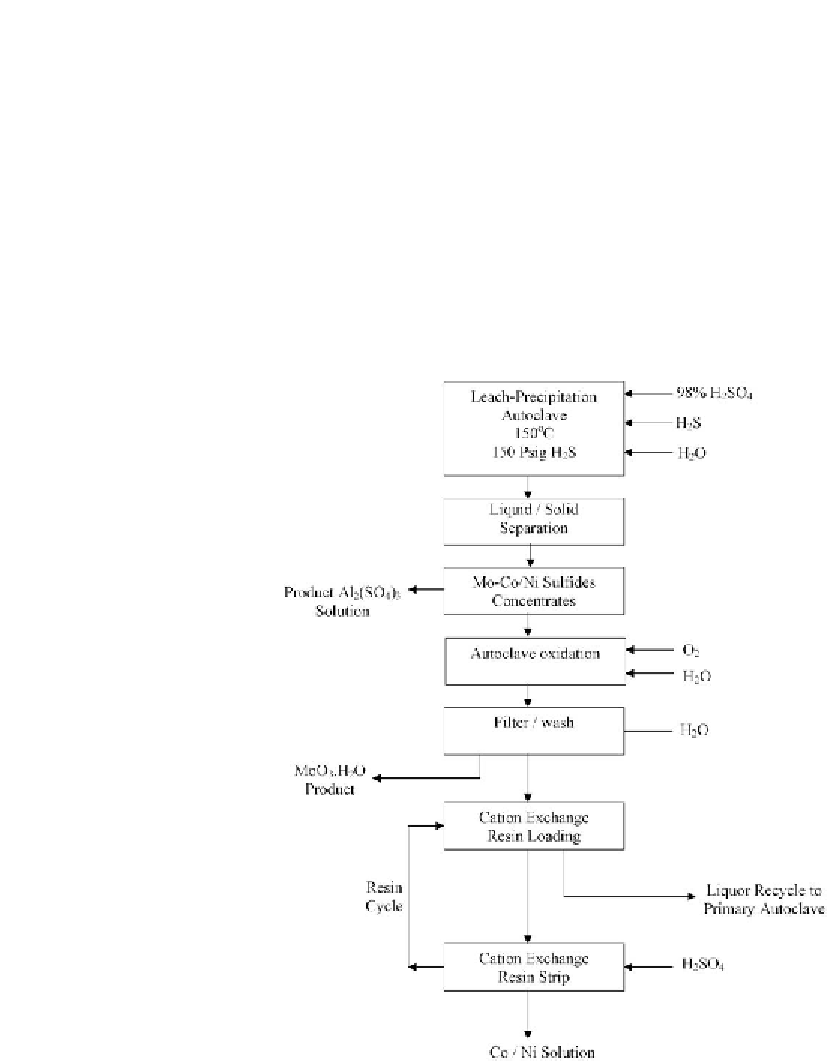
In the patented process developed by Hyatt
[620]
, the spent CoMo/Al
2
O
3
catalyst was treated
with sulfuric acid in the presence of H
2
S under pressure (7.5-15 atm) in an autoclave at
100-200
◦
C. The presence of H
2
S resulted in the precipitation of Mo and Co as sulfides while
the Al
2
O
3
was converted to soluble Al
2
(SO
4
)
3
. The metal sulfides were separated from the
Al
2
(SO
4
)
3
solution and subjected to oxidation under pressure in an autoclave to convert MoS
2
to solid molybdic acid and the CoS to CoSO
4
. The molybdic acid was separated by filtration
from the CoSO
4
solution. The cobalt was recovered by ion exchange. A schematic flow
diagram of the process is shown in
Fig. 11.5 [620]
. The extraction and recovery of valuable
metals, such as Mo and Co together with the alumina as Al
2
(SO
4
)
3
, could be achieved in this
process. Recovery of V is not reported in this acid leaching study.
Figure 11.5: Primary steps for the separation of metals from spent hydrodesulfurization (HDS)
catalyst by H
2
SO
4
in the presence of H
2
S [From ref.
620
. Reprinted with permission].