Environmental Engineering Reference
In-Depth Information
1.92 wt.% MoO
3
, 3.2 wt.% NiO, and 39.6 wt.% Al
2
O
3
, by leaching with the aqueous NH
3
(15-17M) solution at room temperature. Mo and Ni were not leached from the spent catalyst
under these conditions used in the study
[617]
. In a recent study, Yoo et al.
[618]
used
ammonium sulfate solution for leaching Ni from the spent hydrodesulfurization (HDS)
catalyst. About 94% of Ni was leached from the catalyst with 2.6mol/L (NH
4
)
2
SO
4
solution at
the temperature 368 K. The extent of leaching of other metals from the spent catalysts was not
reported in this study.
11.1.1.2 Leaching with Acids
Extraction of the metals present in spent hydroprocessing catalysts by leaching with acids has
been studied extensively by many researchers. It became evident that various types and
concentrations of inorganic acids, such as HCl, H
2
SO
4
, and HNO
3
, have been used as leaching
solutions. Among organic agents, water-soluble organic acids have been attracting most of the
attention. Compared with inorganic acids, aqueous solutions of organic acids ensure an
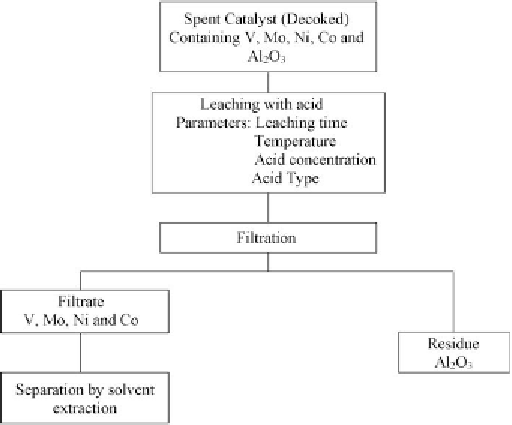
environment requiring much less safety precautions. A generalized scheme for leaching with
acid is shown in
Fig. 11.2
.
11.1.1.2.1 Inorganic acids
In the study of Valverde et al.
[619]
, more than 95% of Co, Ni, and Mo were leached out using
9MH
2
SO
4
at 90
◦
C within 70-90min at stirring rate of 200 rpm. The acid/catalyst ratio,
estimated from the stoichiometric amount of acid required for the conversion of all metals to
Figure 11.2: Metal extraction using acid.